Abstract
Rationale
Previous studies suggest that some behavioral effects of ethanol and morphine are genetically correlated. For example, mice bred for sensitivity (FAST) or insensitivity (SLOW) to the locomotor stimulant effects of ethanol differ in their locomotor response to morphine.
Objective
To evaluate a possible common mechanism for these traits, we examined the effect of naloxone, an opioid receptor antagonist, on ethanol- and morphine-induced locomotion in FAST and SLOW mice, as well as on ethanol-induced locomotion in two heterogeneous stocks of mice.
Method
In experiments 1 and 2, naloxone was given to FAST and SLOW mice 30 min prior to 2 g/kg ethanol or 32 mg/kg morphine, and locomotor activity was measured for 15 min (ethanol) or 30 min (morphine). In experiments 3 and 4, naloxone was administered 30 min prior to 1.25 g/kg ethanol, and locomotor activity was assessed in FAST mice and in a heterogeneous line of mice [Withdrawal Seizure Control (WSC)]. Experiment 5 assessed the effect of naloxone on ethanol-induced stimulation in outbred National Institutes of Health (NIH) Swiss mice.
Results
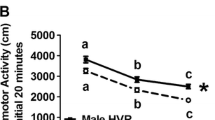
There was no effect of naloxone on the locomotor response to ethanol in FAST, SLOW, WSC, or NIH Swiss mice. However, naloxone did significantly attenuate the locomotor effects of morphine in FAST and SLOW mice.
Conclusions
These results suggest that a common opioidergic mechanism is not responsible for the correlated locomotor responses to ethanol and morphine in FAST and SLOW mice, and that activation of the endogenous opioid system is not critical for the induction of ethanol-induced alterations in activity.





Similar content being viewed by others
References
Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE (1998) Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty. J Neurosci 18:10579–10593
Benjamin D, Grant ER, Pohorecky LA (1993) Naltrexone reverses ethanol-induced dopamine release in the nucleus accumbens in awake, freely moving rats. Brain Res 621:137–140
Bergstrom HC, Palmer AA, Wood RD, Burkhart-Kasch S, McKinnon CS, Phillips TJ (2003) Reverse selection for differential response to the locomotor stimulant effects of ethanol provides evidence for pleiotropic genetic influence on locomotor response to other drugs of abuse. Alcohol Clin Exp Res 27:1535–1547
Boehm SL II, Schafer GL, Phillips TJ, Browman KE, Crabbe JC (2000) Sensitivity to ethanol-induced motor incoordination in 5-HT1B receptor null mutant mice is task-dependent: implications for behavioral assessment of genetically altered mice. Behav Neurosci 114:401–409
Brodie MS, Appel SB (2000) Dopaminergic neurons in the ventral tegmental area of C57BL/6J and DBA/2J mice differ in sensitivity to ethanol excitation. Alcohol Clin Exp Res 24:1120–1124
Brodie MS, Pesold C, Appel SB (1999) Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res 23:1848–1852
Camarini R, Nogueira Pires ML, Calil HM (2000) Involvement of the opioid system in the development and expression of sensitization to the locomotor-activating effect of ethanol. Int J Neuropsychopharmacol 3:303–309
Crabbe JC, Kosobud A, Young ER (1983) Genetic selection for ethanol withdrawal severity: differences in replicate mouse lines. Life Sci 33:955–962
Crabbe JC, Young ER, Deutsch CM, Tam BR, Kosobud A (1987) Mice genetically selected for differences in open-field activity after ethanol. Pharmacol Biochem Behav 27:577–581
Crabbe JC, Deutsch CM, Tam BR, Young ER (1988) Environmental variables differentially affect ethanol-stimulated activity in selectively bred mouse lines. Psychopharmacology 95:103–108
Cui S, Chesson C, Hope R (1993) Genetic variation within and between strains of outbred Swiss mice. Lab Anim 27:116–123
Cunningham CL, Dickinson SD, Okorn DM (1995) Naloxone facilitates extinction but does not affect acquisition or expression of ethanol-induced conditioned place preference. Exp Clin Psychopharmacol 3:330–343
Di Chiara G, Acquas E, Tanda G (1996) Ethanol as a neurochemical surrogate of conventional reinforcers: the dopamine-opioid link. Alcohol 13:13–17
Fromme K, de Wit H, Hutchison KE, Ray L, Corbin WR, Cook TAR, Wall TL, Goldman D (2004) Biological and behavioral markers of alcohol sensitivity. Alcohol Clin Exp Res 28:247–256
Garbutt JC, West SL, Carey TS, Lohr KN, Crews FT (1999) Pharmacological treatment of alcohol dependence: a review of the evidence. JAMA 281:1318–1325
Gevaerd MS, Sultowski ET, Takahashi RN (1999) Combined effects of diethylpropion and alcohol on locomotor activity of mice: participation of the dopaminergic and opioid systems. Braz J Med Biol Res 32:1545–1550
Gianoulakis C (1990) Characterization of the effects of acute ethanol administration on the release of beta-endorphin peptides by the rat hypothalamus. Eur J Pharmacol 180:21–29
Gianoulakis C (2001) Influence of the endogenous opioid system on high alcohol consumption and genetic predisposition to alcoholism. J Psychiatry Neurosci 26:304–318
Gysling K, Wang RY (1983) Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res 277:119–127
Herz A (1997) Endogenous opioid systems and alcohol addiction. Psychopharmacology 129:99–111
Holdstock L, King AC, de Wit H (2000) Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res 24:789–794
Johnson SW, North RA (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12:483–488
Joyce EM, Iversen SD (1979) The effect of morphine applied locally to mesencephalic dopamine cell bodies on spontaneous motor activity in the rat. Neurosci Lett 14:207–212
Kiianmaa K, Hoffman PL, Tabakoff B (1983) Antagonism of the behavioral effects of ethanol by naltrexone in BALB/c, C57BL/6, and DBA/2 mice. Psychopharmacology 79:291–294
King AC, Volpicelli JR, Frazer A, O'Brien CP (1997) Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology 129:15–22
King AC, Houle T, de Wit H, Holdstock L, Schuster A (2002) Biphasic alcohol response differs in heavy vs light drinkers. Alcohol Clin Exp Res 26:827–835
Klitenick MA, DeWitte P, Kalivas PW (1992) Regulation of somatodendritic dopamine release in the ventral tegmental area by opioids and GABA: an in vivo microdialysis study. J Neurosci 12:2623–2632
Kohl RR, Katner JS, Chernet E, McBride WJ (1998) Ethanol and negative feedback regulation of mesolimbic dopamine release in rats. Psychopharmacology 139:79–85
Kranzler HR, Modesto-Lowe V, Van Kirk J (2000) Naltrexone vs nefazodone for treatment of alcohol dependence. A placebo-controlled trial. Neuropsychopharmacology 22:493–503
Larsson A, Svensson L, Söderpalm B, Engel JA (2002) Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol 28:157–167
Latt NC, Jurd S, Houseman J, Wutzke SE (2002) Naltrexone in alcohol dependence: a randomized controlled trial of effectiveness in a standard clinical setting. Med J Aust 176:530–534
Lister RG (1987) The effects of ethanol on exploration in DBA/2 and C57Bl/6 mice. Alcohol 4:17–19
Lynch CJ (1969) The so-called Swiss mouse. Lab Anim Care 19:214–220
Madeira MD, Paula-Barbosa MM (1999) Effects of alcohol on the synthesis and expression of hypothalamic peptides. Brain Res Bull 48:3–22
Marinelli PW, Quirino R, Gianoulakis C (2003) A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology 169:60–67
Matthews RT, German DC (1984) Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience 11:617–625
McClearn GE, Wilson SG, Meredith W (1970) The use of isogenic and heterogenic mouse stocks in behavioral research. In: Thiessen DD (ed) Contributions to behavior-genetic analysis: the mouse as a prototype. Appleton-Century-Crofts, New York, pp 3–22
Mhatre M, Holloway F (2003) μ1-opioid antagonist naloxonazine alters ethanol discrimination and consumption. Alcohol 29:109–116
Middaugh LD, Read E, Boggan WO (1978) Effects of naloxone on ethanol induced alterations of locomotor activity in C57BL/6 mice. Pharmacol Biochem Behav 9:157–160
Middaugh LD, Szumlinski KK, Van Patten Y, Marlowe AL, Kalivas PW (2003) Chronic ethanol consumption by C57BL/6 mice promotes tolerance to its interoceptive cues and increases extracellular dopamine, an effect blocked by naltrexone. Alcohol Clin Exp Res 27:1892–1900
National Institutes of Health (1986) Guidelines for the care and use of laboratory animals (DHEW Publication No. 86–203). U.S. Government Printing Office, Washington, DC
Newlin DB, Thomson JB (1991) Chronic tolerance and sensitization to alcohol in sons of alcoholics. Alcohol Clin Exp Res 15:399–405
Newlin DB, Thomson JB (1999) Chronic tolerance and sensitization to alcohol in sons of alcoholics. II. Replication and reanalysis. Exp Clin Psychopharmacol 7:234–243
Nurmi M, Kiianmaa K, Sinclair JD (1994) Brain ethanol in AA, ANA, and Wistar rats monitored with one-minute microdialysis. Alcohol 11:315–321
O'Brien CP, Volpicelli LA, Volpicelli JR (1996) Naltrexone in the treatment of alcoholism: a clinical review. Alcohol 13:35–39
Olive MF, Koenig HN, Nannini MA, Hodge CW (2001) Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci 21(RC184):1–5
O'Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B (1992) Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry 49:881–887
Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O'Brien CP (2003) A functional polymorphism of the mu-opioid receptor gene in is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology 28:1546–1552
Palmer AA, McKinnon CS, Bergstrom HC, Phillips TJ (2002) Locomotor activity responses to ethanol, other alcohols, and GABA-A acting compounds in forward- and reverse-selected FAST and SLOW mouse lines. Behav Neurosci 116:958–967
Paolone G, Burdino R, Badiani A (2003) Dissociation in the modulatory effects of environmental novelty on the locomotor, analgesic, and eating response to acute and repeated morphine in the rat. Psychopharmacology 166:146–155
Pastor R, Sanchis-Segura C, Aragon CMG (2005) Effect of selective antagonism of mu(1)-, mu (1/2)-, mu(3)-, and delta-opioid receptors on the locomotor-stimulating actions of ethanol. Drug Alcohol Depend 78:289–295
Phillips TJ, Shen EH (1996) Neurochemical bases of locomotion and ethanol stimulant effects. Int Rev Neurobiol 39:243–282
Phillips TJ, Burkhart-Kasch S, Terdal ES, Crabbe JC (1991) Response to selection for ethanol-induced locomotor activation: genetic analyses and selection response characterization. Psychopharmacology 103:557–566
Phillips TJ, Burkhart-Kasch S, Gwiazdon CC, Crabbe JC (1992) Acute sensitivity of FAST and SLOW mice to the effects of abused drugs on locomotor activity. J Pharmacol Exp Ther 261:525–533
Phillips TJ, Shen EH, McKinnon CS, Burkhart-Kasch S, Lessov CN, Palmer AA (2002) Forward, relaxed, and reverse selection for reduced and enhanced sensitivity to ethanol's locomotor stimulant effects in mice. Alcohol Clin Exp Res 26:593–602
Powell KR, Holtzman SG (2001) Parametric evaluation of the development of sensitization to the effects of morphine on locomotor activity. Drug Alcohol Depend 62:83–90
Randall CL, Carpenter JA, Lester D, Friedman HJ (1975) Ethanol-induced mouse strain differences in locomotor activity. Pharmacol Biochem Behav 3:533–535
Reddy BV, Boyadjieva N, Sarkar DK (1995) Effect of ethanol, propanol, butanol, and catalase enzyme blockers on β-endorphin secretion from primary cultures of hypothalamic neurons: evidence for a mediatory role of acetaldehyde in ethanol stimulation of β-endorphin release. Alcohol Clin Exp Res 19:339–344
Sanchis-Segura C, Correa M, Aragon CMG (2000) Lesion on the hypothalamic arcuate nucleus by estradiol valerate results in blockade of ethanol-induced locomotion. Behav Brain Res 114:57–63
Sanchis-Segura C, Pastor R, Aragon CMG (2004) Opposite effects of acute vs chronic naltrexone administration on ethanol-induced locomotion. Behav Brain Res 153:61–67
Schuckit MA, Smith TL (2000) The relationships of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. J Stud Alcohol 61:827–835
Schuckit MA, Smith TL (2001) Correlates of unpredicted outcomes in sons of alcoholics and controls. J Stud Alcohol 62:477–485
Shen EH, Harland RD, Crabbe JC, Phillips TJ (1995) Bidirectional selective breeding for ethanol effects on locomotor activity: characterization of FAST and SLOW mice through selection generation 35. Alcohol Clin Exp Res 19:1234–1245
Shen EH, Dorow J, Huson M, Phillips TJ (1996) Correlated responses to selection in FAST and SLOW mice: effects of ethanol on ataxia, temperature, sedation and withdrawal. Alcohol Clin Exp Res 20:688–696
Shen EH, Dorow J, Harland R, Burkhart-Kasch S, Phillips TJ (1998) Seizure sensitivity and GABAergic modulation of ethanol sensitivity in selectively bred FAST and SLOW mouse lines. J Pharmacol Exp Ther 287:606–615
Smolen TN, Smolen A (1989) Blood and brain ethanol concentrations during absorption and distribution in long-sleep and short-sleep mice. Alcohol 6:33–38
Smoothy R, Berry MS (1985) Time course of the locomotor stimulant and depressant effects of a single low dose of ethanol in mice. Psychopharmacology 85:57–61
Swift RM, Whelihan W, Kuznetsov O, Buongiorno G, Hsuing H (1994) Naltrexone-induced alterations in human ethanol intoxication. Am J Psychiatry 151:1463–1467
Takemori AE, Portoghese PS (1984) Comparative antagonism by naltrexone and naloxone of mu, kappa, and delta agonists. Eur J Pharmacol 104:101–104
Valentinuzzi VS, Buxton OM, Chang AM, Scarbrough K, Ferrari EAM, Takahashi JS, Turek FW (2000) Locomotor response to an open field during C57BL/6J active and inactive phases: differences dependent on conditions of illumination. Physiol Behav 69:269–275
Vezina P, Kalivas PW, Stewart J (1987) Sensitization occurs to the locomotor effects of morphine and the specific μ-opioid receptor agonist, DAGO, administered repeatedly to the ventral tegmental area but not to the nucleus accumbens. Brain Res 417:51–58
Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP (1992) Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry 49:876–880
Williams JT, Christie MJ, Manzoni O (2001) Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev 81:299–343
Acknowledgements
This work was supported by a grant from the Department of Veterans Affairs (TJP), the Agencia Valenciana de Ciencia y Tecnología (RP), and the National Institute on Alcohol Abuse and Alcoholism, grants P50AA10760 (TJP) and T32AA07468 (SEH). The authors gratefully acknowledge the expert technical assistance provided by Sue Burkhart-Kasch, Na Li, and Christina Cotnam, as well as the helpful insight provided by Dr. Chris Cunningham. All experiments reported in this paper were performed in a manner that was consistent with the laws of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holstein, S.E., Pastor, R., Meyer, P.J. et al. Naloxone does not attenuate the locomotor effects of ethanol in FAST, SLOW, or two heterogeneous stocks of mice. Psychopharmacology 182, 277–289 (2005). https://doi.org/10.1007/s00213-005-0066-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0066-8