Abstract
Aims/hypothesis
The aim was to identify potential microRNA (miRNA) biomarkers of type 2 diabetes.
Methods
Controlled studies were retrieved from PubMed to compare miRNA expression profiles of type 2 diabetes and nondiabetic control samples. Meta-analysis under a random effects model was conducted. Subgroup analyses examined tissue specificity and species specificity. Sensitivity analyses were also performed to explain the heterogeneity among studies. Results were represented as log odds ratios (logOR), 95% confidence intervals (CI) and p values after Bonferroni correction.
Results
Among 343 differentially expressed miRNAs in 38 miRNA expression profiling studies published between 1993 and March 2014, only 151 miRNAs were tested by multiple studies, out of which 102 miRNAs were reported to be upregulated or downregulated. Meta-analysis identified 51 significantly dysregulated miRNAs. The top upregulated miRNA was miR-142-3p (logOR 6.4721; 95% CI 4.9537, 7.9904; adjusted p = 4.60 × 10−16). The top downregulated miRNA was miR-126a (logOR 7.5237; 95% CI 4.7159, 10.3316; adjusted p = 3.01 × 10−07). The dysregulation of two miRNAs (miR-199a-3p and miR-223) was highly pancreas-specific and liver-specific. miR-30e was downregulated in patients with type 2 diabetes, while miR-92a was downregulated in animal models of diabetes. In sensitivity analysis, 40 out of 47 miRNAs (85%) were robustly and consistently dysregulated.
Conclusions/interpretation
This meta-analysis confirms that 40 miRNAs are significantly dysregulated in type 2 diabetes. miR-29a, miR-34a, miR-375, miR-103, miR-107, miR-132, miR-142-3p and miR-144 are potential circulating biomarkers of type 2 diabetes. In addition, miR-199a-3p and miR-223 are potential tissue biomarkers of type 2 diabetes.
Similar content being viewed by others
Introduction
Type 2 diabetes is a complex metabolic disorder characterised by insulin resistance [1] that is often undetected until hyperglycaemia is observed [2]. Over time, multiple organ damage can occur, especially to the heart, blood vessels, eyes, kidneys and nerves [3]; thus, exploring novel early biomarkers and therapeutics for diabetes is of great importance. MicroRNAs (miRNAs) are likely to represent early biomarkers of type 2 diabetes that can be used to detect and monitor progression of the disease [4].
miRNAs are a class of small (approximately 22 nucleotides), endogenous, noncoding, highly stable RNAs that regulate gene and protein expression. miRNAs are involved in many biological processes, including cellular differentiation, metabolism and cancer development [5–7], and their modes of dysregulation are linked to many diseases [8]. In studies attempting to identify novel early biomarkers of type 2 diabetes, miRNA expression profiling was often performed in cultured cells, blood or solid tissue samples [9–12]. Large numbers of miRNAs were identified to be differentially expressed, either overexpressed or underexpressed, while only a small number may actually be important signatures or therapeutic targets. Different profiling studies have employed different profiling platforms and different methods. It is, therefore, challenging to determine which miRNAs are potential biomarkers, which miRNAs are tissue-specific, whether circulating miRNAs make the best biomarkers and whether animal models are sufficient for pilot studies. The results of these studies are, however, subject to evaluation by meta-analysis. Recent studies have indicated that the seminal findings from academic laboratories could only be reproduced 11–50% of the time [13, 14]. A survey on data reproducibility of biomedical science also found the problem of data irreproducibility [15]. As literature reviews on miRNAs in type 2 diabetes were purely narrative without any meta-analysis, it came as no surprise that the overall findings were inconsistent (Table 1). Therefore, no consistent and definitive picture is available. For example, a review [16] reported that the regulation of miR-103 in adipose tissue was different according to different experiments, while a second review [18] deemed miR-103 to be downregulated in adipose tissue and a third review [19] found it to be upregulated in adipose tissue. This study aims to fill this gap by using a meta-analysis to identify consistently dysregulated miRNAs that have been shown in reproducible profiling results to be potential biomarkers for type 2 diabetes.
Methods
Search strategies
PubMed was searched for type 2 diabetes miRNA expression profiling studies published between 1993 and 11 March 2014 using the following terms: (‘miRNA’, ‘diabetes’ and ‘expression’ in the title/abstract) or (‘miRNA’, ‘diabetes’ and ‘profile’ in the title/abstract) or (‘miRNA’, ‘diabetes’ and ‘profiling’ in the title/abstract) or (‘microRNA’, ‘diabetes’ and ‘expression’ in the title/abstract) or (‘microRNA’, ‘diabetes’ and ‘profile’ in the title/abstract) or (‘microRNA’, ‘diabetes’ and ‘profiling’ in the title/abstract).
Study selection
Eligible studies had to meet the inclusion criteria: (1) they were miRNA expression profiling studies on patients with type 2 diabetes or on animal models of diabetes; (2) they used diabetic and nondiabetic control samples for comparison; (3) they used miRNA expression arrays; (4) they reported cut-off criteria of differentially expressed miRNAs; and (5) they reported sample sizes. miRNA profiling studies using saliva or urine of type 2 diabetes patients were excluded because we focused on miRNAs in blood, and miRNAs studies in saliva and urine were mostly related to oral cancer [21] and urinary tract cancer [22–24], respectively.
Data extraction and quality assessment
From the full text and supplementary information of each expression profiling study, the following eligibility items were collected and recorded: first author, year of publication, location of study, selection and characteristics of recruited type 2 diabetes patients or animal models of diabetes, miRNA expression profiling platform, sample sizes, tissue types, cut-off criteria of upregulated and downregulated miRNAs and the list of differentially expressed miRNAs and their corresponding fold changes (if available). Quality assessment of microarrays was performed according to the Minimum Information About a Microarray Experiment (MIAME) guideline version 2.0 [25]. Studies involving quantitative PCR (qPCR)-based miRNA arrays were assessed according to the Minimum Information for Publication of Quantitative Real-time PCR Experiments (MIQE) guideline [26], which is equivalent to the MIAME guideline.
Meta-analysis
Extracted data were transferred to the statistical software R (Revolution R Enterprise, version 6.1.0) with the Metafor package (http://cran.r-project.org/web/packages/metafor/index.html, version1.9-5) [27] for meta-analysis under a random effects model. The outcomes are presented as log10 odds ratios (logORs), based on the numbers of dysregulation events in both type 2 diabetes and nondiabetic control samples, with their 95% confidence intervals. Bonferroni corrections were performed on p values. Adjusted p values less than 0.05 were considered significant. When compared with nondiabetic control groups, a significant logOR higher than 1 indicated miRNA upregulation. When compared with the diabetic group, a significant logOR higher than 1 indicated miRNA downregulation. Potential circulating biomarkers should be significantly upregulated or downregulated and detectable in both human and animal blood or in both blood and tissues. Potential tissue biomarkers should be significantly upregulated or downregulated and highly tissue-specific. Differentially expressed miRNAs in type 2 diabetes and nondiabetic control samples were ranked according to the following order of importance: (1) p values; (2) the number of consistent reports; and (3) logOR values.
Subgroup analysis
miRNAs are differentially expressed among tissue types and species, with corresponding overall effects and heterogeneities. Subgroup analyses split the extracted data according to tissue types and species in order to compare miRNA expression profiles among tissue types (blood, muscle, pancreas, liver, etc.) and species (i.e. tissue specificity and species specificity). Studies using serum, plasma or peripheral blood mononuclear cells were classified as blood as they were from blood and aimed to investigate circulating miRNAs. Studies that did not report the pancreatic tissue was whole pancreas or pancreatic islets were pooled with the other studies on pancreatic islets.
Sensitivity analysis
Sensitivity analysis was performed on the sample size to test the robustness of findings. Sample size is a dominant factor that affects precision in determining the overall effects. Thus, the meta-analysis was repeated after excluding the studies for which sample sizes were ten or less.
Results
Included studies and their characteristics
Figure 1 shows the selection process of the studies. A total of 253 potentially relevant studies were identified in PubMed. After removal of duplicated publications and reviews, 38 articles met the eligibility criteria. Details of study characteristics are shown in Tables 2 and 3. Among the 38 included studies, 22 reported only human miRNA expression profiles (Table 2), 15 focused on miRNA expression profiles in animal models (Table 3), and only one was based on both humans and animals. The numbers of differentially expressed miRNAs ranged from 1 to 130, with a median of 10. Fold change information of miRNAs was available in 17 of the 38 studies.
Quality assessment of studies
MIAME guideline 2.0 [25] and MIQE guideline [26] were used to assess study quality. Figure 2 and electronic supplementary material (ESM) Table 1 summarise the quality assessment process. Of the included studies, 74% did not report raw data of hybridisation and 82% of studies did not give sufficient information about experimental design and sample data relationships. For more than 30% of the studies, other key aspects (such as final processed data and array design) were not always fully reported.
Differentially expressed miRNAs
Out of 343 differentially expressed miRNAs reported in the 38 studies that compared type 2 diabetes samples with nondiabetic control samples, 151 miRNAs (44.0%) were reported in at least two substudies. Among the 151 differentially expressed miRNAs, 51 (33.8%) were consistent in their direction of dysregulation (30 were reported to be upregulated and 21 downregulated). In the meta-analysis of dysregulated miRNAs, the dysregulation of these 51 miRNAs was significant, as shown in ESM Tables 2 and 3 (30 upregulated and 21 downregulated). The most reported upregulated miRNA was miR-29a, which was reported in ten substudies with an adjusted p = 3.72 × 10−14. miR-487b (adjusted p = 6.24 × 10−06) was the most reported downregulated miRNA, which was reported in four substudies.
Subgroup analysis
Eighteen of the 38 studies investigated circulating miRNAs, which used plasma, serum, peripheral blood mononuclear cells and whole blood as profiling samples. Six investigated muscle tissue, eight investigated pancreas tissue, three investigated glomeruli tissue, six investigated liver tissue and six investigated adipose tissue. Among the eight pancreas tissue profiling studies, five studies reported using pancreatic islets and the others reported using whole pancreas. Details are shown in Tables 2 and 3. Significant dysregulation of miRNAs in different tissue types is shown in ESM Tables 4–9. Among the dysregulated miRNAs, ten upregulated miRNAs (miR-103, miR-107, miR-132, miR-143, miR-144, miR-21, miR-29a, miR-29b, miR-34a and miR-375) were consistently reported in at least two tissue types, while 36 miRNAs (26 upregulated and ten downregulated) were reported in only one tissue. The other two dysregulated miRNAs (miR-199a-3p and miR-223) were upregulated in one tissue type and downregulated in another (ESM Table 10), which suggests that their regulation is highly tissue-specific.
In subgroup analysis of species, 190 miRNAs were reported in 23 human profiling studies with 54 reported in at least two substudies. Among these 54 miRNAs, 18 were upregulated and seven were downregulated (ESM Table 11), while differential expression of the other 29 was not significant (adjusted p > 0.05).
A total of 241 miRNAs were reported in 16 animal profiling studies with 119 reported in at least two substudies. Among the 119 differentially expressed miRNAs, 35 miRNAs had adjusted p values <0.05 (27 were upregulated and eight were downregulated). Details are shown in ESM Table 12. Comparison of upregulated miRNAs (n = 30) in all profiling studies showed that miR-185 (adjusted p = 6.29 × 10−05), miR-187 (adjusted p = 5.27 × 10−05) and miR-103 (adjusted p = 1.40 × 10−03) were upregulated in human profiling studies, while miR-19a (adjusted p = 9.47 × 10−04), let-7d (adjusted p = 5.53 × 10−06), miR-191 (adjusted p = 1.14 × 10−04), miR-320 (adjusted p = 2.08 × 10−06), miR-27a (adjusted p = 5.36 × 10−04) and miR-29b (adjusted p = 1.57 × 10−07), amongst other miRNAs, were upregulated in animal profiling studies. Comparison of significantly downregulated miRNAs (n = 21) in all profiling studies showed that miR-652 (adjusted p = 8.64 × 10−05) and miR-30e (adjusted p = 5.56 × 10−08) were downregulated in human profiling studies, while miR-92a (adjusted p = 3.15 × 10−04) was downregulated in animal profiling studies. These results indicate that miRNAs might be expressed differently in various species. Human tissue-specific and animal tissue-specific miRNAs had already been analysed. There were no sufficient samples for analysis; thus, the results are not included in this manuscript.
Sensitivity analysis
Sensitivity analysis was conducted to examine the robustness of the findings and to determine what effect sample size had on the overall analysis. Thirty of the 38 included reports had sample sizes greater than ten. We excluded studies where sample size was no more than ten. Analysing the thirty studies identified 47 miRNAs that were significantly differentially expressed, with 29 upregulated and 18 downregulated (ESM Table 13). Forty of the 47 miRNAs (85%) were significantly differentially expressed both in the sensitivity analysis and the overall analysis, while the other seven were not significantly differentially expressed in the overall analysis. This result indicated that the small sample sizes used in miRNA profiling studies may explain some differences in the results of miRNA profiling studies.
Discussion
This study is the first meta-analysis of type 2 diabetes miRNA expression profiling studies and identified specific miRNAs as potential biomarkers of type 2 diabetes. A Venn diagram of miRNA categories and a flow chart of the meta-analysis is shown in Fig. 3.
Flow diagram showing miRNA categories analysed in this meta-analysis. The blocks in the upper part of the diagram show the tissue specificity. The Venn diagram in the lower part summarises the results of the overall analysis, species specificity and sensitivity analyses. miRNAs in orange (n = 40) were found to be robustly and consistently dysregulated in sensitivity analysis. n, number of statistically significant miRNAs
A total of 151 differentially expressed miRNAs were reported in at least two independent substudies. Among these 151 miRNAs, only 51 (34%) were significantly differentially expressed, while 100 (66%) were not. This result is not a surprise as initially we expected that the number of significantly differentially expressed miRNAs might be the same as or less than that of nonsignificantly differentially expressed miRNAs. Several factors may explain the meta-analysis results of the differentially expressed miRNAs, such as publication bias, biological complexity (for example, variations in environmental backgrounds and gene susceptibility), insufficient information about the pattern of miRNA expression in different tissue types (the heterogeneity in different kinds of specimens) and heterogeneous conditions.
The miRNAs exert actions in different tissues, while circulating miRNAs hold much promise as biomarkers of type 2 diabetes. In the tissue subgroup study, 36 miRNAs were only expressed in one tissue, while two (miR-199a-3p and miR-223) were upregulated in one tissue and downregulated in another. Ten miRNAs (miR-103, miR-107, miR-132, miR-143, miR-144, miR-21, miR-29a, miR-29b, miR-34a and miR-375) were consistently reported in multiple tissue types, for example muscle, adipose tissue, liver and pancreas, and seven of these (miR-103, miR-107, miR-132, miR-144, miR-29a, miR-34a and miR-375) were identified in blood. This finding indicates that circulating miRNAs hold much potential as biomarkers of type 2 diabetes [65]. Laterza et al [66] and Kosaka et al [67] have demonstrated how circulating miRNAs may indicate the physiological state at the tissue level. The miRNAs that circulate in the blood are in a stable form and remain stable even after multiple freeze–thaw cycles. They can be detected by minimally invasive techniques [68] and are specific to tissue and disease states [69]. If these miRNAs in circulating blood can serve as biomarkers, they would provide a minimally invasive biomarker approach that would be extremely useful in diagnosing and monitoring type 2 diabetes. Studies on multiple tissues were also analysed (ESM Table 14). Ten miRNAs with significant differential expression were consistently found in liver and adipose tissue among all the studies. The high agreement between the liver and adipose tissue analyses indicate the reliability of liver-specific and adipose-tissue-specific miRNAs. However, more studies are needed to identify miRNAs that are tissue-specific in other tissue types.
miRNAs can be differentially expressed between animals and humans. Although animal studies might be informative about type 2 diabetes indicators in humans, animals are still different from humans. In the species subgroup analysis, the differential expression of some miRNAs was significant in both humans and animals, but other miRNAs, such as let-7d (adjusted p = 5.53 × 10−06) and miR-29b (adjusted p = 1.57 × 10−07), were significantly differentially expressed in animals but not in humans. These results indicate that miRNAs can be differentially expressed in various species and animal models are insufficient to determine indicators of type 2 diabetes in humans. Thus, miRNAs identified as candidates in animal models require verification in humans.
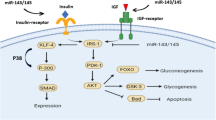
This study identified ten miRNAs (miR-103, miR-107, miR-132, miR-142-3p, miR-144, miR-199a-3p, miR-223, miR-29a, miR-34a and miR-375) as potential biomarkers of type 2 diabetes, including circulating and tissue biomarkers. Circulating biomarkers are preferred to tissue biomarkers due to easier sampling and testing. For extensive validation of tissue biomarkers, animal experiments would be less costly than clinical studies. In cases in which resources for clinical studies are constrained, we would suggest validating the tissue miRNA biomarkers commonly found in both animals and humans before those found only in humans. The potential circulating biomarkers detected in both human and animal blood or in both blood and tissues are shown in Fig. 4. The potential tissue biomarkers were miR-199a-3p and miR-223. Among all the profiling studies, the most frequently reported (ten studies) and upregulated miRNA was miR-29a (adjusted p = 3.72 × 10−14), which was overexpressed together with miR-29c (adjusted p = 5.13 × 10−10) during hyperglycaemia and hyperinsulinaemia. Overexpression of the miR-29 family impairs insulin-stimulated glucose uptake by inhibiting insulin signalling via the Akt signalling pathway [52]. miR-34a (adjusted p = 3.69 × 10−14) ranked second among the upregulated miRNAs in all profiling studies. miR-34a in beta cells increased in response to palmitate, impairing nutrient-induced insulin secretion by repressing vesicle-associated membrane protein 2 (VAMP2) expression [59] and making the beta cells more susceptible to apoptosis [70]. miR-103 and miR-107, which play a central role in regulating insulin sensitivity by targeting caveolin-1 (a regulator of the insulin receptor) [62], were upregulated in the blood of both humans and animals in our analysis. The most upregulated miRNA in human profiling studies was miR-375 (adjusted p = 3.60 × 10−13), which inhibits insulin secretion by repressing its targets myotrophin [71] and phosphoinositide-dependent protein kinase-1 (PDK1) [72]. In both overall analysis and subgroup analysis of species, miR-144 (adjusted p = 4.81 × 10−10) and miR-142-3p (adjusted p = 9.71 × 10−13) were upregulated in human type 2 diabetes blood samples. miR-144 impairs insulin signalling by inhibiting the expression of insulin receptor substrate 1 (IRS1) [31] and miR-142-3p regulates Akt1 (also known as protein kinase Bɑ) in adipogenesis regulation [73, 74]. In the subgroup analysis of tissue types, miR-223 was downregulated in one tissue and upregulated in another. Upregulated miR-223 in cardiomyocytes induces glucose transporter 4 (GLUT4) protein expression to restore normal glucose uptake [36]. miR-132 was upregulated in both blood and liver. miR-132 targets insulin-mediated regulation of CYP2E1 (cytochrome P450, family 2, subfamily E, polypeptide 1), which is involved in hepatic metabolism [75].
Venn diagrams showing some potential miRNA biomarkers of type 2 diabetes. (a) Stepwise selection of miRNAs; (b) commonality of miRNAs among tissues including blood. The prefix ‘miR-’ of miRNA identifiers is omitted. The potential miRNA biomarkers are in the shaded area. The shaded area of Fig. 4b also contains identified potential miRNA biomarkers in both blood and tissues
miR-132 inhibits the expression of LRRFIP1 (leucine-rich repeat (in Flightless 1) interacting protein-1) to block vascular smooth muscle cell proliferation in atherosclerosis and restenosis [76], and miR-144 is reported to regulate haematopoiesis and vascular development by repressing the expression of meis homeobox 1 [77]. Likewise, the miR-29 family, which directly targets Mmp2 (the matrix metalloproteinase 2 gene), Col1a1 (the collagen, type I, alpha 1 gene) and Col3a1 (the collagen, type III, alpha 1 gene), is involved in renal and cardiovascular injury [78]. miR-34a modulates p53 that is related to human atherosclerosis [79]. It also inhibits sirtuin-1 and HMGCR (3-hydroxy-3-methyl-glutaryl-CoA reductase) in nonalcoholic fatty liver disease [80]. miR-375 targets caveolin1, janus kinase 2 (JAK2) and yes-associated protein 1 (YAP1) that are related to cancer [81, 82]. These plausible relationships indicate that miRNAs are associated with not only type 2 diabetes but also the complications of type 2 diabetes. Since specific miRNAs were consistently detected as being dysregulated in multiple studies of (1) both human and animal blood or (2) both blood and tissues, they represent potential biomarkers of type 2 diabetes for extensive validation.
Conclusion
This meta-analysis of type 2 diabetes miRNA expression profiling studies identified 40 significantly dysregulated miRNAs. Eight miRNAs (miR-103, miR-107, miR-132, miR-144, miR-142-3p, miR-29a, miR-34a and miR-375) are potential blood biomarkers, while two miRNAs (miR-199a-3p and miR-223) have high tissue-specific regulation and are potential tissue biomarkers.
Abbreviations
- LogOR:
-
Log odds ratio
- MIAME:
-
Minimum information about a microarray experiment
- MIQE:
-
Minimum information for publication of quantitative real-time PCR experiments
- miRNA:
-
MicroRNA
- qPCR:
-
Quantitative PCR
References
Kahn SE (2001) Clinical review 135: the importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 86:4047–4058
Karolina DS, Armugam A, Sepramaniam S, Jeyaseelan K (2012) MiRNAs and diabetes mellitus. Expert Rev Endocrinol Metab 7:281–300
Winer N, Sowers JR (2004) Epidemiology of diabetes. J Clin Pharmacol 44:397–405
Shantikumar S, Caporali A, Emanueli C (2012) Role of microRNAs in diabetes and its cardiovascular complications. Cardiovasc Res 93:583–593
Carrington JC, Ambros V (2003) Role of microRNAs in plant and animal development. Science 301:336–338
Suárez Y, Sessa WC (2009) MicroRNAs as novel regulators of angiogenesis. Circ Res 104:442–454
Van Rooij E, Olson EN (2011) Modulation of K-ras-dependent lung tumorigenesis by microRNA-21. Cancer Cell 18:282–293
Fabian MR, Sonenberg N, Filipowicz W (2010) Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem 79:351–379
Bagge A, Clausen TR, Larsen S et al (2012) MicroRNA-29a is up-regulated in beta-cells by glucose and decreases glucose-stimulated insulin secretion. Biochem Biophys Res Commun 426:266–272
Locke JM, Harries LW (2012) MicroRNA expression profiling of human islets from individuals with and without type 2 diabetes: promises and pitfalls. Biochem Soc Trans 40:800–803
Karolina DS, Tavintharan S, Armugam A et al (2012) Circulating miRNA profiles in patients with metabolic syndrome. J Clin Endocrinol Metab 97:E2271–E2276
Liu J, Liu W, Ying H et al (2012) Analysis of microRNA expression profile induced by AICAR in mouse hepatocytes. Gene 512:364–372
Prinz F, Schlange T, Asadullah K (2011) Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov 10:712
Begley CG, Ellis LM (2012) Raise standards for preclinical cancer research. Nature 483:531–533
Mobley A, Linder SK, Braeuer R et al (2013) A survey on data reproducibility in cancer research provides insights into our limited ability to translate findings from the laboratory to the clinic. PLoS One 8:e63221
Guay C, Roggli E, Nesca V et al (2011) Diabetes mellitus, a microRNA-related disease? Transl Res 157:253–264
Guay C, Jacovetti C, Nesca V (2012) Emerging roles of non-coding RNAs in pancreatic β-cell function and dysfunction. Diabetes Obes Metab 14:12–21
Hamar P (2012) Role of regulatory microRNAs in type 2 diabetes mellitus related inflammation. Nucleic Acid Ther 22:289–294
McClelland AD, Kantharidis P (2014) MicroRNA in the development of diabetic complications. Clin Sci (Lond) 126:95–110
Natarajan R, Putta S, Kato M (2012) MicroRNAs and diabetic complications. J Cardiovasc Transl Res 5:413–422
Park N, Zhou H, Elashoff D (2009) Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin Cancer Res 15:5473–5477
Hanke M, Hoefig K, Merz H et al (2010) A robust methodology to study urine microRNA as tumor marker: microRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol Oncol 28:655–661
Weber JA, Baxter DH, Zhang S et al (2010) The microRNA spectrum in 12 body fluids. Clin Chem 56:1733–1741
Yamada Y, Enokida H, Kojima S et al (2011) MiR-96 and miR-183 detection in urine serve as potential tumor markers of urothelial carcinoma: correlation with stage and grade, and comparison with urinary cytology. Cancer Sci 102:522–529
The Functional Genomics Data Society (1991) Minimum information about a microarray experiment - MIAME 2.0. Available from www.mged.org/Workgroups/MIAME/miame_2.0.html. Accessed 26 Apr 2007
Bustin SA, Benes V, Garson JA et al (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36:1–48
Balasubramanyam M, Aravind S, Gokulakrishnan K et al (2011) Impaired miR-146a expression links subclinical inflammation and insulin resistance in type 2 diabetes. Mol Cell Biochem 351:197–205
Corral-Fernández NE, Salgado-Bustamante M, Martínez-Leija ME et al (2013) Dysregulated miR-155 expression in peripheral blood mononuclear cells from patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 121:347–353
Gallagher IJ, Scheele C, Keller P et al (2010) Integration of microRNA changes in vivo identifies novel molecular features of muscle insulin resistance in type 2 diabetes. Genome Med 2:9
Karolina DS, Armugam A, Tavintharan S et al (2011) MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in type 2 diabetes mellitus. PLoS One 6:e22839
Klöting N, Berthold S, Kovacs P et al (2009) MicroRNA expression in human omental and subcutaneous adipose tissue. PLoS One 4:e4699
Kong L, Han W, Jiang X et al (2010) Expression and clinical significance of peripheral miR-34a during the onset of type 2 diabetes. J Shangdong Univ Health Sci 48:2–4
Kong L, Zhu J, Han W et al (2011) Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol 48:61–69
Locke JM, da Silva Xavier G, Dawe HR et al (2014) Increased expression of miR-187 in human islets from individuals with type 2 diabetes is associated with reduced glucose-stimulated insulin secretion. Diabetologia 57:122–128
Lu H, Buchan RJ, Cook SA (2010) MicroRNA-223 regulates Glut4 expression and cardiomyocyte glucose metabolism. Cardiovasc Res 86:410–420
Meng S, Cao J-T, Zhang B et al (2012) Downregulation of microRNA-126 in endothelial progenitor cells from diabetes patients impairs their functional properties via target gene Spred-1. J Mol Cell Cardiol 53:64–72
Ortega FJ, Moreno-Navarrete JM, Pardo G et al (2010) MiRNA expression profile of human subcutaneous adipose and during adipocyte differentiation. PLoS One 5:e9022
Ortega FJ, Mercader JM, Moreno-Navarrete JM et al (2014) Profiling of circulating microRNAs reveals common microRNAs linked to type 2 diabetes that change with insulin sensitization. Diabetes Care 37:1375–1383
Pescador N, Pérez-Barba M, Ibarra JM et al (2013) Serum circulating microRNA profiling for identification of potential type 2 diabetes and obesity biomarkers. PLoS One 8:e77251
Rong Y, Bao W, Shan Z et al (2013) Increased microRNA-146a levels in plasma of patients with newly diagnosed type 2 diabetes mellitus. PLoS One 8:e73272
Spinetti G, Fortunato O, Caporali A et al (2013) MicroRNA-15a and microRNA-16 impair human circulating proangiogenic cell functions and are increased in the proangiogenic cells and serum of patients with critical limb ischemia. Circ Res 112:335–346
Sun K, Chang X, Yin L et al (2014) Expression and DNA methylation status of microRNA-375 in patients with type 2 diabetes mellitus. Mol Med Rep 9:967–972
Wang H, Lin Y, Zhou Y et al (2012) Circulatory level of microRNA 92a in patients of stable angina pectoris with diabetes mellitus. China J Mod Med 22:26–29
Wang X, Sundquist J, Zöller B et al (2014) Determination of 14 circulating microRNAs in Swedes and Iraqis with and without type 2 diabetes mellitus. PLoS One 9:e86792
Zampetaki A, Kiechl S, Drozdov I et al (2010) Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res 107:810–817
Zhang T, Lv C, Li L et al (2013) Plasma miR-126 is a potential biomarker for early prediction of type 2 diabetes mellitus in susceptible individuals. Biomed Res Int 2013:761617
Zhao H, Guan J, Lee H-M et al (2010) Up-regulated pancreatic tissue microRNA-375 associates with human type 2 diabetes through beta-cell deficit and islet amyloid deposition. Pancreas 39:843–846
Zhou J, Peng R, Li T et al (2013) A potentially functional polymorphism in the regulatory region of let-7a-2 is associated with an increased risk for diabetic nephropathy. Gene 527:456–461
Chettimada S, Ata H, Rawat DK et al (2014) Contractile protein expression is upregulated by reactive oxygen species in aorta of Goto-Kakizaki rat. Am J Physiol Heart Circ Physiol 306:H214–H224
Esguerra JLS, Bolmeson C, Cilio CM, Eliasson L (2011) Differential glucose-regulation of microRNAs in pancreatic islets of non-obese type 2 diabetes model Goto-Kakizaki rat. PLoS One 6:e18613
He A, Zhu L, Gupta N et al (2007) Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol Endocrinol 21:2785–2794
Herrera BM, Lockstone HE, Taylor JM et al (2009) MicroRNA-125a is over-expressed in insulin target tissues in a spontaneous rat model of type 2 diabetes. BMC Med Genomics 2:54
Herrera BM, Lockstone HE, Taylor JM et al (2010) Global microRNA expression profiles in insulin target tissues in a spontaneous rat model of type 2 diabetes. Diabetologia 53:1099–1109
Jordan SD, Krüger M, Willmes DM et al (2011) Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat Cell Biol 13:434–446
Kato M, Putta S, Wang M et al (2009) TGF-β activates Akt kinase via a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol 11:881–889
Kato M, Wang L, Putta S et al (2010) Post-transcriptional up-regulation of Tsc-22 by Ybx1, a target of miR-216a, mediates TGF-β-induced collagen expression in kidney cells. J Biol Chem 285:34004–34015
Kaur K, Pandey AK, Srivastava S et al (2011) Comprehensive miRNome and in silico analyses identify the Wnt signaling pathway to be altered in the diabetic liver. Mol Biosyst 7:3234–3244
Lovis P, Roggli E, Laybutt DR et al (2008) Alterations in microRNA expression contribute to fatty acid-induced pancreatic β-cell dysfunction. Diabetes 57:2728–2736
Nesca V, Guay C, Jacovetti C et al (2013) Identification of particular groups of microRNAs that positively or negatively impact on beta cell function in obese models of type 2 diabetes. Diabetologia 56:2203–2212
Poy MN, Hausser J, Trajkovski M et al (2009) MiR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc Natl Acad Sci U S A 106:5813–5818
Trajkovski M, Hausser J, Soutschek J et al (2011) MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 474:649–653
Suvh M, Villeneuve LM, Kato M et al (2010) Enhanced levels of microRNA-125b in vascular smooth muscle cells of diabetic db/db mice lead to increased inflammatory gene expression by targeting the histone. Diabetes 59:2904–2915
Zhang Z, Peng H, Chen J et al (2009) MicroRNA-21 protects from mesangial cell proliferation induced by diabetic nephropathy in db/db mice. FEBS Lett 583:2009–2014
Gilad S, Meiri E, Yogev Y et al (2008) Serum microRNAs are promising novel biomarkers. PLoS One 3:e3148
Laterza OF, Lim L, Garrett-Engele PW et al (2009) Plasma microRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem 55:1977–1983
Kosaka N, Iguchi H, Yoshioka Y et al (2010) Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 285:17442–17452
Cho WCS (2011) Circulating microRNAs as minimally invasive biomarkers for cancer theragnosis and prognosis. Front Genet 2:1–6
Chen X, Ba Y, Ma L et al (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 18:997–1006
Roggli E, Britan A, Gattesco S et al (2010) Involvement of microRNAs in the cytotoxic effects exerted by proinflammatory cytokines on pancreatic β-cells. Diabetes 59:978–986
Poy MN, Eliasson L, Krutzfeldt J et al (2004) A pancreatic islet-specific microRNA regulates insulin secretion. Nature 432:226–230
El Ouaamari A, Baroukh N, Martens GA et al (2008) MiR-375 targets 3-phosphoinositide-dependent protein kinase-1 and regulates glucose-induced biological responses in pancreatic β-cells. Diabetes 57:2708–2717
Chartoumpekis DV, Zaravinos A, Ziros PG et al (2012) Differential expression of microRNAs in adipose tissue after long-term high-fat diet-induced obesity in mice. PLoS One 7:e34872
Yun S-J, Kim E-K, Tucker DF et al (2008) Isoform-specific regulation of adipocyte differentiation by Akt/protein kinase B alpha. Biochem Biophys Res Commun 371:138–143
Shukla U, Tumma N, Gratsch T et al (2013) Insights into insulin-mediated regulation of CYP2E1: miR-132/-212 targeting of CYP2E1 and role of phosphatidylinositol 3-kinase, Akt (protein kinase B), mammalian target of rapamycin signaling in regulating miR-132/-212 and miR-122/-181a expression in primary cultured rat hepatocytes. Drug Metab Dispos 41:1769–1777
Choe N, Kwon J-S, Kim J-R et al (2013) The microRNA miR-132 targets Lrrfip1 to block vascular smooth muscle cell proliferation and neointimal hyperplasia. Atherosclerosis 229:348–355
Su Z, Si W, Li L et al (2014) MiR-144 regulates hematopoiesis and vascular development by targeting meis1 during zebrafish development. Int J Biochem Cell Biol 49:53–63
Kriegel AJ, Liu Y, Fang Y et al (2012) The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics 44:237–244
Raitoharju E, Lyytikäinen L-P, Levula M et al (2011) MiR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis 219:211–217
Min HK, Kapoor A, Fuchs M et al (2013) Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty disease. Cell Metab 15:665–674
Chen B, Li H, Zeng X et al (2012) Roles of microRNA on cancer cell metabolism. J Transl Med 10:228
Kinoshita T, Hanazawa T, Nohata N et al (2012) The functional significance of microRNA-375 in human squamous cell carcinoma: aberrant expression and effects on cancer pathways. J Hum Genet 57:556–563
Funding
The work of HZ and SL has been sponsored by two research grants (MYRG190-Y3-L3-ICMS11-LSW and MYRG2014-00117-ICMS-QRCM) received from the University of Macau.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
SL is the guarantor of this work and conceived the meta-analysis. SL and HZ designed the protocol. HZ searched the databases, assessed and selected studies, and extracted and analysed the data from the selected studies according to the eligibility criteria. SL and HZ interpreted the data and drafted a report on the findings. SL revised the manuscript for submission. All authors read and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
(PDF 34 kb)
ESM Table 2
(PDF 114 kb)
ESM Table 3
(PDF 107 kb)
ESM Table 4
(PDF 110 kb)
ESM Table 5
(PDF 96 kb)
ESM Table 6
(PDF 101 kb)
ESM Table 7
(PDF 93 kb)
ESM Table 8
(PDF 104 kb)
ESM Table 9
(PDF 94 kb)
ESM Table 10
(PDF 231 kb)
ESM Table 11
(PDF 111 kb)
ESM Table 12
(PDF 119 kb)
ESM Table 13
(PDF 52.4 kb)
ESM Table 14
(PDF 179 kb)
Rights and permissions
About this article
Cite this article
Zhu, H., Leung, S.W. Identification of microRNA biomarkers in type 2 diabetes: a meta-analysis of controlled profiling studies. Diabetologia 58, 900–911 (2015). https://doi.org/10.1007/s00125-015-3510-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-015-3510-2