Abstract
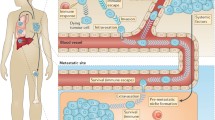
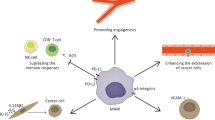
The metastatic process is characterized by a complex series of sequential steps involving constant interactions (mutual “cross-talks”) of metastasized tumor cells with their microenvironment (lymphocyte, macrophages, endothelial cells, etc.) in target organs. These interactions determine the outcome of metastasis (either the eradication of metastatic cells or their increased proliferation and invasion). Recently developed methods of tumor and host cell analysis at the molecular level allow better elucidation of molecular mechanisms of metastasis and of immune mechanisms involved in antitumor responses. Direct modulation of these processes will probably increase the success of clinical cancer treatment. Here we review data (a) on the expression of some costimulatory (MHC class II, CD80, sialoadhesin) and adhesion (LFA1, ICAM-1, VLA-4) molecules on both metastasized tumor cells and host cells and (b) on the production of a cytotoxic molecule, nitric oxide, by in situ activated Kupffer and endothelial cells in the process of liver metastasis. This study was performed with well-characterized murine ESbL T lymphoma cells transduced with the bacterial lacZ gene, which allows detection and quantification of metastases at the single cell level throughout lymphoma growth and metastasis. Experimental results are discussed in the context of recent literature.
Similar content being viewed by others
Abbreviations
- APC :
-
Antigen-presenting cells
- hCRP :
-
Human C-reactive protein
- ICAM :
-
Intercellular adhesion molecule
- IFN :
-
Interferon
- IL :
-
Interleukin
- iNOS :
-
Inducible NO synthase
- LFA :
-
Leukocyte function associated antigen
- SER :
-
Sheep erythrocyte receptor
- TA :
-
Tumor-associated rejection antigens
- TNF :
-
Tumor necrosis factor
- VCAM :
-
Vascular cell adhesion molecule
- VLA :
-
Very late activated antigen
References
Weiss L (1985) Principles of metastasis. Academic, Orlando
Fidler IJ, Balch KI (1987) The biology of cancer metastasis and implications for therapy. Curr Probl Surg 24:131–209
Nicolson GL (1993) Cancer progression and growth: relationship of paracrine and autocrine growth mechanisms to organ preference of metastasis. Exp Cell Res 204:171–180
Mareel MM, Van Roy FM, Bracke ME (1993) How and when do tumor cells metastasize? Crit Rev Oncogenesis 4:559–594
Price JE (1994) Host-tumor interactions in the progression of breast cancer metastasis. In Vivo 8:145–154
Nicolson GL (1989) Organ specificity of tumor metastasis: role of preferential adhesion, invasion, and growth of malignant cells at specific secondary sites. Cancer Metastasis Rev 7:143–188
Fidler IJ (1990) Host and tumour factors in cancer metastasis. Eur J Clin Invest 20:481–486
Whiteside TL, Herberman RB (1992) Extravasation of antitumor effector cells. Invasion Metastasis 12:128–146
Paget S (1889) The distribution of secondary growths in cancer of the breast. Lancet I:571–573
Schirrmacher V (1992) Immunity and metastasis: In situ activation of protective T-cells by virus modified cancer vaccines. Cancer Surv 13:129–154
Den Otter W (1986) Immune surveillance and natural resistance: an evaluation. Cancer Immunol Immunother 21:85–92
Whitworth PW, Pak CC, Esgro J, Kleinerman ES, Fidler IJ (1990) Macrophages and cancer. Cancer Metastasis Rev 8:319–351
Belloni PN, Tressler RJ (1990) Microvascular endothelial cell heterogeneity: interactions with leucocytes and tumor cells. Cancer Metastasis Rev 8:353–389
Miller FR (1993) Immune mechanisms in the sequential steps of metastasis. Crit Rev Oncogenesis 4:293–311
Rosenberg SA (1991) Immunotherapy and gene therapy of cancer. Cancer Res 51:5074–5079
Fidler IJ (1991) Orthotopic implantation of human colon carcinomas into nude mice provides a valuable model for the biology and therapy of metastasis. Cancer Metastasis Rev 10:229–243
Radinsky R, Fidler IJ (1992) Regulation of tumor cell growth at organ-specific metastases. In Vivo 6:325–331
Pallesen G, Hamilton-Dutoit SJ, Zhou X (1993) The association of Epstein-Barr virus (EBV) with T cell lymphoproliferations and Hodgkin's disease: two developments in the EBV field. Adv Cancer Res 62:179–239
Armitage JO (1993) Treatment of non-Hodgkin's lymphoma. N Engl J Med 328:1023–1030
Rudders R, Levin A, Jespersen D, Zacks J, Delellis R, Ranger A, Kronitis T (1992) Crossreacting human lymphoma idiotypes. Blood 80:1039–1044
Benke R, Lang E, Komitowski D, Muto S, Schirrmacher V (1988) Changes in tumor cell adhesiveness affecting speed of dissemination and mode of metastatic growth. Invasion Metastasis 8:159–176
Schackert G, Fidler IJ (1988) Site-specific metastasis of mouse melanomas and a fibrosarcoma in the brain or meninges of syngeneic animals. Cancer Res 48:3478–3484
Krüger A, Schirrmacher V, von Hoegen P (1994) Scattered micrometastasis visualized at the single-cell level: detection and re-isolation of lacZ-labeled metastasized lymphoma cells. Int J Cancer 58:275–284
Lampson LA, Lampson MA, Dunne AD (1993) Exploiting the lacZ reporter gene for quantitative analysis of disseminated tumor growth within the brain: use of the lacZ gene product as a tumor antigen, for evaluation of antigenic modulation, and to facilitate image analysis of tumor growth in situ. Cancer Res 53:176–182
Dooley TP, Stamp-Cole M, Ouding RJ (1993) Evaluation of a nude mouse tumor model using β-galactosidase-expressing melanoma cells. Lab Anim Sci 43:48–57
Kurebayashi J, MsLeskey SW, Johnson MD, Lippman ME, Dickson RB, Kern FG (1993) Quantitative demonstration of spontaneous metastasis by MCF-7 human breast cancer cells cotransfected with fibroblast growth factor 4 and LacZ. Cancer Res 53:2178–2187
Schirrmacher V, Beckhove P, Krüger A, Rocha M, Umansky V, Fichtner KP, Hull WE, Zangemeister-Wittke U, Griesbach A, Jurianz K, von Hoegen P (1995) Effective immune rejection of advanced metastasized cancer. Int J Oncol 6:505–521
Schirrmacher V, Schild HJ, Gückel B, von Hoegen P (1992) Tumor specific CTL response requiring interactions of four different cell types and dual recognition of MHC class I and class II restricted tumor antigens. Immunl Cell Biol 71:311–326
Schirrmacher V, Zangemeister-Wittke U (1994) γ-Irradiation suppresses T cell mediated protective immunity against a metastatic tumor in the afferent phase of the immune response but enhances it in the efferent phase when given before immune cell transfer. Int J Oncol 4:335–346
Schild HJ, Kyewski B, von Hoegen P, Schirrmacher V (1987) CD4+ helper T cells are required for resistance to a highly metastatic murine tumour. Eur J Immunol 17:1863–1866
Gückel B, Berek C, Lutz M, Altevogt P, Schirrmacher V, Kyewski B (1991) Anti-CD2 antibodies induce T cell unresponsiveness in vivo. J Exp Med 174:957–967
Schirrmacher V, Leidig S, Griesbach A (1991) In situ activation of syngeneic tumour-specific cytotoxic T lymphocytes: intra-pinna immunization followed by restimulation in the peritoneal cavity. Cancer Immunol Immunother 33:299–306
Krüger A, Umansky V, Rocha M, Hacker HJ, Schirrmacher V, von Hoegen P (1994) Pattern and load of spontaneous liver metastasis dependent on host immune status studied with a lacZ transduced lymphoma. Blood 84:3166–3174
Rocha M, Krüger A, Umansky V, von Hoegen P, Naor D, Schirrmacher V (1996) Dynamic expression-changes in vivo of adhesion and costimulatory molecules determine load and pattern of lymphoma liver metastasis. Clin Cancer Res (in press)
Plautz GE, Yang ZY, Wu BY, Gao X, Huang L, Nabel GJ (1994) Immunotherapy of malignancy by in vivo transfer into tumors. Proc Natl Acad Sci USA 90:4645–4649
Schirrmacher V, Fogel M, Russmann E, Bosslet K, Altevogt P, Beck L (1982) Antigenic variation in cancer metastasis: immune escape versus immune control. Cancer Met Rev 1:241–274
Cromme FV, Airey J, Heemels MT, Ploegh HL, Keating PJ, Stern PL, Meijer CJ, Walboomers JM (1994) Loss of transporter protein, encoded by the TAP-1 gene, is highly correlated with loss of HLA expression in cervical carcinomas. J Exp Med 179:335–340
Guardiola J, Maffei A (1993) Control of MHC class II gene expression in autoimmune, infectious and neoplastic disease. Crit Rev Immunol 13:247–268
Janeway CA Jr, Bottomly K (1994) Signals and signs for lymphocyte responses. Cell 76:275–285
Azuma M, Yssel H, Phillips JJ, Spits H, Lanier LL (1993) Functional expression of B7/BB1 on activated T lymphocytes. J Exp Med 177:845–850
Chen L, Ashe S, Brady WA, Hellstrom I, Hellstrom KE, Ledbetter JA, McGovan P, Linsley PS (1992) Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell 71:1093–1098
Chen L, Linsley PS, Hellstrom KE (1993) Costimulation of T cells for tumor immunity. Immunol Today 14:483–486
Towsend SE, Allison JP (1993) Tumor rejection after direct Costimulation of CD8+ T cells by B7-transfected melanoma cells. Science 259:368–371
Baskar S, Nabavi N, Glimcher LH, Rosenberg S (1993) Tumor cells expressing major histocompatibility complex class II and B7 activation molecules stimulate potent tumor-specific immunity. J Immunother 14:209–215
Becker JC, Brabletz T, Czerny C, Termeer C, Brocker EB (1993) Tumor escape mechanisms for immunosurveillance: induction of unresponsiveness in a specific MHC-restricted CD4+ human T cell clone by the autologous MHC class II+ melanoma. Int Immunol 5:1501–1508
Graf L, Koch N, Schirrmacher V (1985) Expression of Ia antigens in a murine T-lymphoma variant. Mol Immunol 22:1371–1377
Semino C, Ferlazzo G, Pietra G, Pasquetti W, Repetto L, Rosso R, Mariani M, Melioli G (1993) Heterogeneity of the alpha-interferon mediated overexpression of class I and class II major histocompatibility complex molecules in primary cultured cancer cells. J. Biol Regul Homeost Agents 7:99–104
Crocker PR, Gordon S (1986) Properties and distribution of a lectin-like hemagglutinin differentially expressed by stromal tissue macropages. J Exp Med 164:1862–1875
Crocker PR, Gordon S (1989) Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J Exp Med 169:1333–1346
Crocker PR, Kelm S, Dubois C, Martin B, McWilliam AS, Shotton D, Paulson JC, Gordon S (1991) Purification and properties of sialoadhesin, a sialic acid-binding receptor of murine tissue macrophages. EMBO J 10:1661–1669
Crocker PR, Mucklow S, Bouckson V, McWilliam A, Willis AC, Gordon S, Milon G, Kelm S, Bradfield P (1994) Sialoadhesin, a macrophage sialic acid binding receptor for haemopoietic cells with 17 immunoglobulin-like domains. EMBO J 13:4490–4503
Van den Berg TK, Breve JJP, Damoiseaux JGMC, Döpp EA, Kelm S, Crocker PR, Dijkstra CD, Kraal G (1992) Sialoadhesin on macrophages: its identification as a lymphocyte adhesion molecule. J Exp Med 176:647–655
Umansky V, Beckhove P, Rocha M, Krüger A, Crocker PR, Schirrmacher V (1996) A role for sialoadhesin-positive tissue macrophages in host resistance to lymphoma metastasis in vivo. Immunology 87:303–309
Damoiseaux JGMC, Döpp EA, Beelen RHJ, Dijkstra CD (1989) Rat bone marrow and monocyte cultures: influence of culture time and lymphokines on the expression of macrophage differentiation antigens. J. Leukocyte Biol 46:246–253
Damoiseaux JGMC, Huitinga I, Döpp EA, Dijkstra CD (1992) Expression of the ED3 antigen on rat macrophages in relation to experimental autoimmune diseases. Immunobiology 184:311–320
Crocker PR, Hill M, Gordon S (1988) Regulation of a murine macrophage haemagglutinin (sheep erythrocyte receptor) by a species-restricted serum factor. Immunology 65:515–522
McWilliam AS, Tree P, Gordon S (1992) Interleukin 4 regulates induction of sialoadhesin, the macrophage sialic acidspecific receptor. Proc Natl Acad Sci USA 89:10522–10526
Doyle AG, Herbein G, Montaner L, Minty AJ, Caput D, Ferrara P, Gordon S (1994) Interleukin-13 alters the activation state of murine macrophages in vitro: comparison with interleukin-4 and interferon-γ. Eur J Immunol 24, 1441–1445
Klein T, Döffinger R, Pepis MB, Rüther U, Kyewski B (1995) Tolerance and immunity to the inducible self antigen C-reactive protein in transgenic mice. Eur J Immunol 25:3489–3495
Albelda SM (1993) Biology of disease. Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest 68:4–17
Roos E, Roosien FF (1987) Involvement of leucocyte function-asocciated antigen-1 (LFA-1) in the invasion of hepatocyte cultures by lymphoma and T-cell hybridoma cells. J Cell Biol 105:553–557
Grander B, Wang P, Einhorn S (1994) Gamma-IFN induced cell adhesion in chronic myelogenous leukemia cells. Leukemia 8:299–304
Stauder R, Greil R, Schulz TF, Gattringer C, Radaskiewicz T, Dierich MP, Huber H (1989) Expression of leucocyte function-associated antigen-1 and 7F7 antigen, an adhesion molecule related to intercellular adhesion molecules-1 (ICAM-1) in non-Hodgkin lymphomas and leukaemias: possible influence of growth and leukaemic behaviour. Clin Exp Immunol 77:234–238
Harning R, Myers C, Merluzzi VJ (1993) Monoclonal antibodies to lymphocyte function-associated antigen-1 inhibit invasion of human lymphoma and metastasis of murine lymphoma. Clin Exp Metast 11:337–342
Zahalka MA, Ökon E, Naor D (1993) Blocking lymphoma invasiveness with a monoclonal antibody directed against the β-chain of the leucocyte adhesion molecule (CD18). J Immunol 150:4466–4477
Clayberger C, Wright A, Medeiros LJ, Koller TD, Link MP, Smith SD, Warnke RA, Krensky AM (1987) Absence of cell surface LFA-1 as a mechanism of escape from immunosurveillance. Lancet II:533–536
Koyama S, Ebihara T, Fukao K (1992) Expression of intercellular adhesion molecule-1 (ICAM-1) during development of invasion and/or metastasis of gastric carcinoma. J Cancer Res Clin Oncol 118:609–614
Dolcetti R, Maestro R, Gasparotto D, Rizzo S, Boiocchi M (1992) Adhesion molecule expression does not influence the leukemic behaviour of murine T-cell lymphomas. Leukemia 6:101S-105S
Johnson JP, Stade BG, Holzman B, Schwable W, Riethmuller G (1989) De novo expression of intercellular adhesion molecule in melanoma correlates with increased risk of metastasis. Proc Natl Acad Sci USA 86:641–644
Schirren CA, Volpel H, Hoffmann JC, Henning SW, Qiao L, Autschbach F, Dengler TJ, Dohner H, Meuer SC (1992) Biological response modifiers render tumor cells susceptible to autologous effector mechanisms by influencing adhesion receptors. Leuk Lymphoma 10:25–33
Gonzalez-Amaro R, Garcia-Monzon C, Garcia-Buey L, Moreno-Otero R, Alonso JL, Yague E, Pivel JP, Lopez Cabrera M, Fernandez Ruiz E (1994) Induction of tumor necrosis factor alpha production by human hepatocytes in chronic viral hepatitis. J Exp Med 179:841–848
Jurianz K (1995) Molekulare Analyse der Induktion tumorspezifische Immunantworten: Vergleich von Tumor-resistenz und Suszeptibilität in einem Maus Tumor Modellsystem. PhD Thesis, University of Heidelberg
Dustin ML, Staunton DE, Springer TA (1988) Supergene families meet in the immune system. Immunol Today 9:213–215
Kim SJ, Kim NS, Lee JL (1993) Effect of cytokines on the expression of cell adhesion molecules and on the adhesion of melanoma cells to endothelial cells. J Korean Med Sci 8:41–52
Grander B, Wang P, Einhorn S (1994) Gamma-IFN induced cell adhesion in chronic myelogenous leukemia cells. Leukemia 8:299–304
Hansen AB, Bouchelouche PN, Lillevang ST, Andersen CB (1994) Interferon-gamma increases cellular calcium ion concentration and inositol 1,4,5-trisphosphate formation in human renal carcinoma cells: relation to ICAM-1 antigen expression. Br J Cancer 69:291–298
Hutchins D, Steel M (1994) Regulation of ICAM-1 (CD54) expression in human breast cancer lines by interleukin 6 and fibroblast-derived factors. Int J Cancer 58:80–84
Swerlick RA, Lawley TJ (1993) Role of microvascular endothelial cells in inflammation. J Invest Dermatol 100:111S-115S
Antonia SJJ, Uchida S, Cohen S, Cohen MC (1989) Attachment of tumor cells to endothelial monolayers: detection of surface molecules involved in cell-binding. Clin Immunol Immunopathol 53:281–285
Rice GE, Beviclaqua MP (1989) An inducible endothelial surface glycoprotein mediates melanoma adhesion. Science 246:1303–1306
Hemler ME, Elices MJ, Parker C, Takada Y (1990) Structure of the integrin VLA-4 and its cell-cell and cell-matrix adhesion functions. Immunol Rev 114:45–65
Freedmann AS, Saporito L, Rhynhart K, Morimoto C, Nadler LM (1994) Adhesion of follicular lymphoid germinal centers: a potential mechanism of tumor cell homing following autologous transplantation. Leuk Lymphoma 13:47–52
Van Riet I, Van Camp B (1993) The involvement of adhesion molecules in the biology of multiple myeloma. Leuk Lymphoma 9:441–452
Juneja HS, Schmalsteig FC, Lee S, Chen J (1993) Vascular cell adhesion molecule-1 and VLA-4 are obligatory adhesion proteins in the heterotypic adherence between human leukemia/lymphoma cells and marrow stromal cells. Exp Hematol 21:444–450
Qian F, Vaux DL, Weissmann IL (1994) Expression of the integrin α4β1 on melanoma cells can inhibit the invasive stage of metastasis formation. Cell 77:335–347
Okahara H, Yagita H, Miyake K, Okumura K (1994) Involvement of very late activation antigen 4 (VLA-4) and vascular cell adhesion molcule (VCAM-1) in tumor necrosis factor alpha enhancement of experimental metastasis. Cancer Res 54:3233–3236
Garofalo A, Chiviri RGS, Foglieni C, Pigott R, Mortarini R, Martin-Padura I, Anichini A, Gearing AI, Sanchez-Madrid F, Dejana E, Giavazzi, R (1995) Involvement of very late antigen 4 integrin on melanoma in interleukin 1-augmented experimental metastasis. Cancer Res 55:414–419
Rocha M, Umansky V, Schirrmacher V, Elices MJ (1996) In situ downregulation of VLA-4 integrin cell surface expression during lymphoma growth and liver metastasis. (submitted)
Bouwens L, Baekeland M, Wisse E (1984) Importance of local proliferation in the expanding Kupffer cell population of rat liver after zymosan stimulation and partial hepatectomy. Hepatology 4:213–219
Phillips NC (1989) Kupffer cells and liver metastases. Optimization and limitation of activation of tumoricidal activity. Cancer Metastasis Rev 8:231–252
Zhang W, Arii S, Sassaoki T, Adachi Y, Funaki N, Higashitsuji H, Fujita S, Furutani M, Mise M, Ishiguro S (1993) The role of Kupffer cells in the surveillance of tumor growth in the liver. J Surg Res 55:140–146
McCuskey PA, Kan Z, Wallace S (1994) An electron microscopy study of Kupffer cells in livers of mice having Friend erythroleukemia hepatic metastases. Clin Exp Metastasis 12:416–426
Kan Z, Ivancev K, Lunderquist A, McCuskey PA, McCuskey RS, Wallace S (1995) In vivo microscopy of hepatic metastases: dynamic observation of tumor cell invasion and interaction with Kupffer cells. Hepatology 21:487–494
Heuff G, Oldenburg HSA, Boutkan H, Visser JJ, Beelen RHJ, Van Rooijen N, Dijkstra CD, Meyer S (1993) Enhanced tumour growth in the rat liver after selective elimination of Kupffer cells. Cancer Immunol Immunother 37:125–130
Hibbs JB, Taintor R, Vavrin Z, Rachlin EM (1988) Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun 157:87–94
Stuehr DJ, Nathan CF (1989) Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med 169:1543–1555
Moncada S, Palmer RJ, Higgs EA (1991) Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev 43:109–134
Marletta MA, Yoon PS, Lyengar R, Leaf CD, Wishnok JS (1988) Macrophage oxidation of -arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry 27:8706–8711
Kwon NS, Stuehr DJ, Nathan CF (1991) Inhibition of tumor cell ribonucleotide ribonuclease by macrophage-derived nitric oxide. J Exp Med 174:761–767
Umansky V, Rocha M, Krüger A, von Hoegen P, Schirrmacher V (1995) In situ activated macrophages are involved in host resistance to lymphoma metastasis by production of nitric oxide. Int J Oncol 7:33–40
Fidler U, Schroit AJ (1988) Recognition and destruction of neoplastic cells by activated macrophages: discrimination of altered self. Biochem Biophys Acta 948:151–174
Takeshita M, Sumiyoshi Y, Masuda Y, Ohshima K, Yoshida T, Kikuchi M, Muller H (1993) Cytokine (interleukin-1 alpha, interleukin-1 beta, tumor necrosis factor alpha, and interleukin-6) possessing in lymph nodes of malignant lymphoma. Pathol Res Pract 189:18–25
Aono K, Isobe K, Nakashima I, Kondo S, Miyachi M, Nimura Y (1994) Kupffer cells cytotoxicity against hepatoma cells is related to nitric oxide. Biochem Biophys Res Commun 201:1175–1181
Kurose I, Miura S, Fukumura D, Yonei Y, Saito H, Tada S, Suematsu M, Tsuchiya M (1993) Nitric oxide mediates Kupffer cell-induced reduction of mitochondrial energization in hepatoma cells. A comparison with oxidative burst. Cancer Res 53:2676–2682
Karnovsky ML, Lazdins JK (1978) Biochemical criteria for activated macrophages. J Immunol 121:809–813
Xie K, Huang S, Dong Z, Gutman M, Fidler IJ (1995) Direct correlation between expression of endogenous inducible nitric oxide synthase and regression of M5076 reticulum cell sarcoma hepatic metastases in mice treated with liposomes containing lipopeptide CGP 31362. Cancer Res 55:3123–3131
Senger DR, Perruzzi CA, Gracey CF, Papadopoulos A, Tenen DG (1988) Secreted phosphoproteins associated with neoplastic transformation: close homology with plasma proteins cleaved during blood coagulation. Cancer Res 48:5770–5774
Denhardt DT, Chambers AF (1994) Overcoming obstacles to metastasis-defenses against host defenses: osteopontin (OPN) as a shield against attack by cytotoxic host cells. J Cell Biochem 56:1–4
Feng B, Rollo EE, Denhardt DT (1995) Osteopontin (OPN) may facilitate metastasis by protecting cells from macrophage NO-mediated cytotoxicity: evidence from cell lines downregulated for OPN expression by a targeted ribozyme. Clin Exp Met 13:453–462
Weber GF, Ashkar S, Glimcher MJ, Cantor H (1996) Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Scince 271:509–512
Schirrmacher V, Fogel M, Russmann E, Bosslet K, Altevogt P, Beck L (1982) Antigenic variation in cancer metastasis: Immune escape versus immune control. Cancer Met Rev 1:241–274
Von Hoegen P, Weber E and Schirrmacher V (1986) Modification of tumor cells by a low dose of Newcastle disease virus. I. Augmentation of the tumor-specific T cell response in the absence of an antiviral response. Eur J Immunol 18:1159–1164
Schirrmacher V (1989) Immunobiology and immunotherapy of cancer metastases. Interdise Sci Rev 14:291–303
Thorbecke GJ, Silberberg-Sinakin I, Flotte TJ (1980) Langerhans cells as macrophages in skin and lymphoid organs. J Invest Dermatol 75:32–43
Palmer RMJ, Ferrige AG, Moncada S (1987) Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327:524–526
Li L, Kilbourn RG, Adams J, Fidler IJ (1991) Role of nitric oxide in lysis of tumor cells by cytokine-activated endothelial cells. Cancer Res 51:2531–2535
Li L, Nicolson GL, Fidler IJ (1991) Direct in vitro lysis of metastatic tumor cells by cytokine-activated murine vascular endothelial cells. Cancer Res 51:245–254
Rocha M, Krüger A, van Rooijen N, Schirrmacher V, Umansky V (1995) Liver endothelial cells participate in T cell dependent host resistance to lymphoma metastasis by production of nitric oxide in vivo. Int J Cancer 63:405–411
Estrada C, Gomez C, Martin C, Moncada S, Gonzalez C (1992) Nitric oxide medites tumor necrois factor-alpha cytotoxicity in endothelial cells. Biochem Biophys Res Commun 186:475–82
Van Roojien N, Sanders AM (1994) Liposome mediated depletion of macrophages: mechansim of action, preparation of liposomes and applications. J Immunol Method 174:83–93
Bogers WMJM, Stadt RK, Jansenn DJ, Van Rooijen N, Van Es LA, Dajha MR (1991) Kupffer cell depletion in vivo results in preferential elimination of IgG aggregates and immune complexes via specific Fc receptors on rat liver endothelial cells. Clin Exp Immunol 86:328–333
Radinsky R, Beltran PJ, Tsan R, Zhang R, Cone RD, Fidler IJ (1995) Transcriptional induction of the melanocyte-stimulating hormone receptor in brain metastases of murine K-1735 melanoma. Cancer Res 55:141–148
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Umansky, V., Schirrmacher, V. & Rocha, M. New insights into tumor-host interactions in lymphoma metastasis. J Mol Med 74, 353–363 (1996). https://doi.org/10.1007/BF00210630
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00210630