Abstract
Bone formation and degradation are perfectly coordinated. In case of an imbalance of these processes diseases occur associated with exaggerated formation of new bone or bone loss as in osteoporosis. Most studies investigating osteoporosis either focus on osteoblast or osteoclast function and differentiation. Both processes have been suggested to be affected by reactive oxygen species (ROS). Besides a potentially harmful role of ROS, these small molecules are important second messengers. The family of NADPH oxidases produces ROS in a controlled and targeted manner, to specifically regulate signal transduction. This review will highlight the role of reactive oxygen species in bone cell differentiation and bone-loss associated disease with a special focus on osteoporosis and NADPH oxidases as specialized sources of ROS.



Similar content being viewed by others
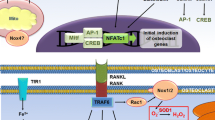
Abbreviations
- Steap:
-
Six-transmembrane epithelial antigen of prostate
- IL:
-
Interleukin
- TNF:
-
Tumour necrosis factor
- IFN:
-
Interferon
- TGF:
-
Transforming growth factor
- NAC:
-
N-acetyl-cysteine
- RANK:
-
Receptor activator of nuclear factor (NF)-κB–receptor
- RANKL:
-
Receptor activator of NF-κB ligand
- OPG:
-
Osteoprotegerin
- BMP-2:
-
Bone morphogenic protein 2
References
Altindag O, Erel O, Soran N et al (2008) Total oxidative/anti-oxidative status and relation to bone mineral density in osteoporosis. Rheumatol Int 28(4):317–321. doi:10.1007/s00296-007-0452-0
Sendur OF, Turan Y, Tastaban E et al (2009) Antioxidant status in patients with osteoporosis: a controlled study. Joint Bone Spine 76(5):514–518. doi:10.1016/j.jbspin.2009.02.005
LeBoff MS, Narweker R, LaCroix A et al (2009) Homocysteine levels and risk of hip fracture in postmenopausal women. J Clin Endocrinol Metabol 94(4):1207–1213. doi:10.1210/jc.2008-1777
Schröder K (2014) NADPH oxidases in redox regulation of cell adhesion and migration. Antioxid Redox Signal 20(13):2043–2058. doi:10.1089/ars.2013.5633
Schröder K, Kohnen A, Aicher A et al (2009) NADPH oxidase Nox2 is required for hypoxia-induced mobilization of endothelial progenitor cells. Circ Res 105(6):537–544. doi:10.1161/CIRCRESAHA.109.205138
Adachi T, Togashi H, Suzuki A et al (2005) NAD(P)H oxidase plays a crucial role in PDGF-induced proliferation of hepatic stellate cells. Hepatology 41(6):1272–1281. doi:10.1002/hep.20719
Lee C, Lin C, Lee I et al (2011) Activation and induction of cytosolic phospholipase A2 by TNF-α mediated through Nox2, MAPKs, NF-κB, and p300 in human tracheal smooth muscle cells. J Cell Physiol 226(8):2103–2114. doi:10.1002/jcp.22537
Wilkinson-Berka JL, Rana I, Armani R et al (2013) Reactive oxygen species, Nox and angiotensin II in angiogenesis: implications for retinopathy. Clin Sci 124(10):597–615. doi:10.1042/CS20120212
Miyano K, Sumimoto H (2007) Role of the small GTPase Rac in p22phox-dependent NADPH oxidases. Biochimie 89(9):1133–1144. doi:10.1016/j.biochi.2007.05.003
Banfi B, Clark RA, Steger K et al (2003) Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem 278(6):3510–3513. doi:10.1074/jbc.C200613200
Ueno N, Takeya R, Miyano K et al (2005) The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: its regulation by oxidase organizers and activators. J Biol Chem 280(24):23328–23339. doi:10.1074/jbc.M414548200
Helmcke I, Heumüller S, Tikkanen R et al (2009) Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal 11(6):1279–1287. doi:10.1089/ARS.2008.2383
Al Ghouleh I, Frazziano G, Rodriguez AI et al (2013) Aquaporin 1, Nox1, and Ask1 mediate oxidant-induced smooth muscle cell hypertrophy. Cardiovasc Res 97(1):134–142. doi:10.1093/cvr/cvs295
Katsuyama M, Matsuno K, Yabe-Nishimura C (2012) Physiological roles of NOX/NADPH oxidase, the superoxide-generating enzyme. J Clin Biochem Nutr 50(1):9–22. doi:10.3164/jcbn.11-06SR
Bedard K, Krause K (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87(1):245–313. doi:10.1152/physrev.00044.2005
Bedard K, Jaquet V, Krause K (2012) NOX5: from basic biology to signaling and disease. Free Radic Biol Med 52(4):725–734. doi:10.1016/j.freeradbiomed.2011.11.023
Lyle AN, Deshpande NN, Taniyama Y et al (2009) Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res 105(3):249–259. doi:10.1161/CIRCRESAHA.109.193722
Sturrock A, Cahill B, Norman K et al (2006) Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290(4):L661–L673. doi:10.1152/ajplung.00269.2005
Clempus RE, Sorescu D, Dikalova AE et al (2007) Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol 27(1):42–48. doi:10.1161/01.ATV.0000251500.94478.18
Goettsch C, Babelova A, Trummer O et al (2013) NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. J Clin Invest 123(11):4731–4738. doi:10.1172/JCI67603
Hecker L, Vittal R, Jones T et al (2009) NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15(9):1077–1081. doi:10.1038/nm.2005
Li J, Stouffs M, Serrander L et al (2006) The NADPH oxidase NOX4 drives cardiac differentiation: role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell 17(9):3978–3988. doi:10.1091/mbc.E05-06-0532
Takac I, Schröder K, Zhang L et al (2011) The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286(15):13304–13313. doi:10.1074/jbc.M110.192138
Paffenholz R, Bergstrom RA, Pasutto F et al (2004) Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev 18(5):486–491. doi:10.1101/gad.1172504
Kao C, Tai L, Chiou S et al (2010) Resveratrol promotes osteogenic differentiation and protects against dexamethasone damage in murine induced pluripotent stem cells. Stem Cells Dev 19(2):247–258. doi:10.1089/scd.2009.0186
Manolagas SC, Parfitt AM (2010) What old means to bone. Trends Endocrinol Metab 21(6):369–374. doi:10.1016/j.tem.2010.01.010
Lane D, Matte I, Laplante C et al (2013) Osteoprotegerin (OPG) activates integrin, focal adhesion kinase (FAK), and Akt signaling in ovarian cancer cells to attenuate TRAIL-induced apoptosis. J Ovarian Res 6(1):82. doi:10.1186/1757-2215-6-82
Boyce BF (2013) Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res 92(10):860–867. doi:10.1177/0022034513500306
Huang JC, Sakata T, Pfleger LL et al (2004) PTH Differentially Regulates Expression of RANKL and OPG. J Bone Miner Res 19(2):235–244. doi:10.1359/JBMR.0301226
Hjortnaes J, Butcher J, Figueiredo J et al (2010) Arterial and aortic valve calcification inversely correlates with osteoporotic bone remodelling: a role for inflammation. Eur Heart J 31(16):1975–1984. doi:10.1093/eurheartj/ehq237
McCarthy I (2006) The physiology of bone blood flow: a review. J Bone Joint Surg Am 88((suppl_2)):4. doi:10.2106/JBJS.F.00890
Zaidi M (2007) Skeletal remodeling in health and disease. Nat Med 13(7):791–801. doi:10.1038/nm1593
Pinzone JJ, Hall BM, Thudi NK et al (2009) The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood 113(3):517–525. doi:10.1182/blood-2008-03-145169
Jian J, Pelle E, Huang X (2009) Iron and menopause: does increased iron affect the health of postmenopausal women? Antioxid Redox Signal 11(12):2939–2943. doi:10.1089/ARS.2009.2576
Zarjou A, Jeney V, Arosio P et al (2010) Ferritin ferroxidase activity: a potent inhibitor of osteogenesis. J Bone Miner Res 25(1):164–172. doi:10.1359/jbmr.091002
Yang X, Chen-Barrett Y, Arosio P et al (1998) Reaction paths of iron oxidation and hydrolysis in horse spleen and recombinant human ferritins. Biochemistry 37(27):9743–9750. doi:10.1021/bi973128a
Tsay J, Yang Z, Ross FP et al (2010) Bone loss caused by iron overload in a murine model: importance of oxidative stress. Blood 116(14):2582–2589. doi:10.1182/blood-2009-12-260083
Jia P, Xu YJ, Zhang ZL et al (2012) Ferric ion could facilitate osteoclast differentiation and bone resorption through the production of reactive oxygen species. J Orthop Res 30(11):1843–1852. doi:10.1002/jor.22133
Nojiri H, Saita Y, Morikawa D et al (2011) Cytoplasmic superoxide causes bone fragility owing to low-turnover osteoporosis and impaired collagen cross-linking. J Bone Miner Res 26(11):2682–2694. doi:10.1002/jbmr.489
Monnier VM, Glomb M, Elgawish A et al (1996) The mechanism of collagen cross-linking in diabetes: a puzzle nearing resolution. Diabetes 45(Suppl 3):S67–S72
Morikawa D, Nojiri H, Saita Y et al (2013) Cytoplasmic reactive oxygen species and SOD1 regulate bone mass during mechanical unloading. J Bone Miner Res 28(11):2368–2380. doi:10.1002/jbmr.1981
Dernbach E, Urbich C, Brandes RP et al (2004) Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood 104(12):3591–3597. doi:10.1182/blood-2003-12-4103
Fatokun AA, Stone TW, Smith RA (2008) Responses of differentiated MC3T3-E1 osteoblast-like cells to reactive oxygen species. Eur J Pharmacol 587(1–3):35–41. doi:10.1016/j.ejphar.2008.03.024
Cai W, Zhang M, Yu Y et al (2013) Treatment with hydrogen molecule alleviates TNFα-induced cell injury in osteoblast. Mol Cell Biochem 373(1–2):1–9. doi:10.1007/s11010-012-1450-4
Choi EM (2011) Luteolin protects osteoblastic MC3T3-E1 cells from antimycin A-induced cytotoxicity through the improved mitochondrial function and activation of PI3K/Akt/CREB. Toxicol In Vitro 25(8):1671–1679. doi:10.1016/j.tiv.2011.07.004
Choi EM, Lee YS (2012) Protective effect of apocynin on antimycin A-induced cell damage in osteoblastic MC3T3-E1 cells. J Appl Toxicol 32(9):714–721. doi:10.1002/jat.1689
Choi EM, Lee YS (2011) Involvement of PI3K/Akt/CREB and redox changes in mitochondrial defect of osteoblastic MC3T3-E1 cells. Toxicol In Vitro 25(5):1085–1088. doi:10.1016/j.tiv.2011.03.022
Mandal CC, Ganapathy S, Gorin Y et al (2011) Reactive oxygen species derived from Nox4 mediate BMP2 gene transcription and osteoblast differentiation. Biochem J 433(2):393–402. doi:10.1042/BJ20100357
Schröder K, Wandzioch K, Helmcke I et al (2009) Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol 29(2):239–245. doi:10.1161/ATVBAHA.108.174219
van Driel M, van Leeuwen Johannes P T M (2014) Vitamin D endocrine system and osteoblasts. Bonekey Rep 3:493. doi:10.1038/bonekey.2013.227
Somjen D, Katzburg S, Grafi-Cohen M et al (2011) Vitamin D metabolites and analogs induce lipoxygenase mRNA expression and activity as well as reactive oxygen species (ROS) production in human bone cell line. J Steroid Biochem Mol Biol 123(1–2):85–89. doi:10.1016/j.jsbmb.2010.11.010
Boyan BD, Bonewald LF, Sylvia VL et al (2002) Evidence for distinct membrane receptors for 1 alpha,25-(OH)(2)D(3) and 24R,25-(OH)(2)D(3) in osteoblasts. Steroids 67(3–4):235–246
Atkins GJ, Kostakis P, Pan B et al (2003) RANKL expression is related to the differentiation state of human osteoblasts. J Bone Miner Res 18(6):1088–1098. doi:10.1359/jbmr.2003.18.6.1088
Tilyard MW, Spears GF, Thomson J et al (1992) Treatment of postmenopausal osteoporosis with calcitriol or calcium. N Engl J Med 326(6):357–362. doi:10.1056/NEJM199202063260601
Eisman JA, Bouillon R (2014) Vitamin D: direct effects of vitamin D metabolites on bone: lessons from genetically modified mice. Bonekey Rep 3:499. doi:10.1038/bonekey.2013.233
Yin H, Shi Z, Yu Y et al (2012) Protection against osteoporosis by statins is linked to a reduction of oxidative stress and restoration of nitric oxide formation in aged and ovariectomized rats. Eur J Pharmacol 674(2–3):200–206. doi:10.1016/j.ejphar.2011.11.024
Huang W, Shang W, Li D et al (2012) Simvastatin protects osteoblast against H2O2-induced oxidative damage via inhibiting the upregulation of Nox4. Mol Cell Biochem 360(1–2):71–77. doi:10.1007/s11010-011-1045-5
Walsh MC, Choi Y (2003) Biology of the TRANCE axis. Cytokine Growth Factor Rev 14(3–4):251–263
Lomaga MA, Yeh WC, Sarosi I et al (1999) TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev 13(8):1015–1024
Kadono Y, Okada F, Perchonock C et al (2005) Strength of TRAF6 signalling determines osteoclastogenesis. EMBO Rep 6(2):171–176. doi:10.1038/sj.embor.7400345
Chandel NS, Schumacker PT, Arch RH (2001) Reactive oxygen species are downstream products of TRAF-mediated signal transduction. J Biol Chem 276(46):42728–42736. doi:10.1074/jbc.M103074200
Zhou J, Ye S, Fujiwara T et al (2013) Steap4 plays a critical role in osteoclastogenesis in vitro by regulating cellular iron/reactive oxygen species (ROS) levels and cAMP response element-binding protein (CREB) activation. J Biol Chem 288(42):30064–30074. doi:10.1074/jbc.M113.478750
Srinivasan S, Koenigstein A, Joseph J et al (2010) Role of mitochondrial reactive oxygen species in osteoclast differentiation. Ann N Y Acad Sci 1192:245–252. doi:10.1111/j.1749-6632.2009.05377.x
Lee NK, Choi YG, Baik JY et al (2005) A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106(3):852–859. doi:10.1182/blood-2004-09-3662
Sasaki H, Yamamoto H, Tominaga K et al (2009) NADPH oxidase-derived reactive oxygen species are essential for differentiation of a mouse macrophage cell line (RAW264.7) into osteoclasts. J Med Invest 56(1–2):33–41
Sasaki H, Yamamoto H, Tominaga K et al (2009) Receptor activator of nuclear factor-kappaB ligand-induced mouse osteoclast differentiation is associated with switching between NADPH oxidase homologues. Free Radic Biol Med 47(2):189–199. doi:10.1016/j.freeradbiomed.2009.04.025
Nakanishi A, Hie M, Iitsuka N et al (2013) A crucial role for reactive oxygen species in macrophage colony-stimulating factor-induced RANK expression in osteoclastic differentiation. Int J Mol Med 31(4):874–880. doi:10.3892/ijmm.2013.1258
Daiber A (2010) Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta 1797(6–7):897–906. doi:10.1016/j.bbabio.2010.01.032
Yang S, Madyastha P, Bingel S et al (2001) A new superoxide-generating oxidase in murine osteoclasts. J Biol Chem 276(8):5452–5458. doi:10.1074/jbc.M001004200
Yang S, Zhang Y, Ries W et al (2004) Expression of Nox4 in osteoclasts. J Cell Biochem 92(2):238–248. doi:10.1002/jcb.20048
Schröder K, Zhang M, Benkhoff S et al (2012) Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 110(9):1217–1225. doi:10.1161/CIRCRESAHA.112.267054
van Phan T, Sul O, Ke K et al (2013) Carbon monoxide protects against ovariectomy-induced bone loss by inhibiting osteoclastogenesis. Biochem Pharmacol 85(8):1145–1152. doi:10.1016/j.bcp.2013.01.014
Hyeon S, Lee H, Yang Y et al (2013) Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free Radic Biol Med 65:789–799. doi:10.1016/j.freeradbiomed.2013.08.005
Gurney EP, Nachtigall MJ, Nachtigall LE et al (2014) The Women’s Health Initiative trial and related studies: 10 years later: a clinician’s view. J Steroid Biochem Mol Biol 142C:4–11. doi:10.1016/j.jsbmb.2013.10.009
Almeida M, Han L, Martin-Millan M et al (2007) Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282(37):27285–27297. doi:10.1074/jbc.M702810200
Muthusami S, Ramachandran I, Muthusamy B et al (2005) Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin Chim Acta 360(1–2):81–86. doi:10.1016/j.cccn.2005.04.014
Lean JM, Jagger CJ, Kirstein B et al (2005) Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology 146(2):728–735. doi:10.1210/en.2004-1021
Jagger CJ, Lean JM, Davies JT et al (2005) Tumor necrosis factor-alpha mediates osteopenia caused by depletion of antioxidants. Endocrinology 146(1):113–118. doi:10.1210/en.2004-1058
Gower BA, Casazza K (2013) Divergent effects of obesity on bone health. J Clin Densitom 16(4):450–454. doi:10.1016/j.jocd.2013.08.010
Parhami F, Garfinkel A, Demer LL (2000) Role of lipids in osteoporosis. Arterioscler Thromb Vasc Biol 20(11):2346–2348. doi:10.1161/01.ATV.20.11.2346
Iqbal J, Sun L, Cao J et al (2013) Smoke carcinogens cause bone loss through the aryl hydrocarbon receptor and induction of Cyp1 enzymes. Proc Natl Acad Sci 110(27):11115–11120. doi:10.1073/pnas.1220919110
Mikosch P (2014) Alcohol and bone. Wien Med Wochenschr 164(1–2):15–24. doi:10.1007/s10354-013-0258-5
Saville PD (1965) Changes in bone mass with age and alcoholism. J Bone Joint Surg Am 47:492–499
Chen J, Haley RL, Hidestrand M et al (2006) Estradiol protects against ethanol-induced bone loss by inhibiting up-regulation of receptor activator of nuclear factor-kappaB ligand in osteoblasts. J Pharmacol Exp Ther 319(3):1182–1190. doi:10.1124/jpet.106.109454
Chen J, Lazarenko OP, Shankar K et al (2011) Inhibition of NADPH oxidases prevents chronic ethanol-induced bone loss in female rats. J Pharmacol Exp Ther 336(3):734–742. doi:10.1124/jpet.110.175091
Sumi D, Hayashi T, Matsui-Hirai H et al (2003) 17beta-estradiol inhibits NADPH oxidase activity through the regulation of p47phox mRNA and protein expression in THP-1 cells. Biochim Biophys Acta 1640(2–3):113–118
Mercer KE, Sims CR, Yang CS et al (2014) Loss of functional NADPH oxidase 2 protects against alcohol-induced bone resorption in female p47phox−/− mice. Alcohol Clin Exp Res 38(3):672–682. doi:10.1111/acer.12305
Chen J, Shankar K, Nagarajan S et al (2008) Protective effects of estradiol on ethanol-induced bone loss involve inhibition of reactive oxygen species generation in osteoblasts and downstream activation of the extracellular signal-regulated kinase/signal transducer and activator of transcription 3/receptor activator of nuclear factor-kappaB ligand signaling cascade. J Pharmacol Exp Ther 324(1):50–59. doi:10.1124/jpet.107.130351
Wosje KS, Kalkwarf HJ (2007) Bone density in relation to alcohol intake among men and women in the United States. Osteoporos Int 18(3):391–400. doi:10.1007/s00198-006-0249-0
Oršolić N, Goluža E, Đikić D et al (2013) Role of flavonoids on oxidative stress and mineral contents in the retinoic acid-induced bone loss model of rat. Eur J Nutr. doi:10.1007/s00394-013-0622-7
Kotake S, Udagawa N, Takahashi N et al (1999) IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 103(9):1345–1352. doi:10.1172/JCI5703
Huang H, Kim HJ, Chang E et al (2009) IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death Differ 16(10):1332–1343. doi:10.1038/cdd.2009.74
Hultqvist M, Olofsson P, Holmberg J et al (2004) Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci 101(34):12646–12651. doi:10.1073/pnas.0403831101
Gelderman KA, Hultqvist M, Pizzolla A et al (2007) Macrophages suppress T cell responses and arthritis development in mice by producing reactive oxygen species. J Clin Invest 117(10):3020–3028. doi:10.1172/JCI31935
Engdahl C, Lindholm C, Stubelius A et al (2013) Periarticular bone loss in antigen-induced arthritis. Arthritis Rheum 65(11):2857–2865. doi:10.1002/art.38114
Cedergren J, Forslund T, Sundqvist T et al (2007) Intracellular oxidative activation in synovial fluid neutrophils from patients with rheumatoid arthritis but not from other arthritis patients. J Rheumatol 34(11):2162–2170
Ralston SH (1997) The michael mason prize essay 1997, Nitric oxide and bone: what a gas! Br J Rheumatol 36(8):831–838
Korkmaz Y, Baumann MA, Schroder H et al (2004) Localization of the NO-cGMP signaling pathway molecules, NOS III-phosphorylation sites, ERK1/2, and Akt/PKB in osteoclasts. J Periodontol 75(8):1119–1125. doi:10.1902/jop.2004.75.8.1119
Armour KE, Ralston SH (1998) Estrogen upregulates endothelial constitutive nitric oxide synthase expression in human osteoblast-like cells. Endocrinology 139(2):799–802. doi:10.1210/endo.139.2.5910
Zaman G, Pitsillides AA, Rawlinson SC et al (1999) Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res 14(7):1123–1131. doi:10.1359/jbmr.1999.14.7.1123
Aguirre J, Buttery L, O’Shaughnessy M et al (2001) Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am J Pathol 158(1):247–257. doi:10.1016/S0002-9440(10)63963-6
Felson DT, Zhang Y, Hannan MT et al (1993) Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res 8(5):567–573. doi:10.1002/jbmr.5650080507
Thomas T, Burguera B (2002) Is leptin the link between fat and bone mass? J Bone Miner Res 17(9):1563–1569. doi:10.1359/jbmr.2002.17.9.1563
Turner RT, Kalra SP, Wong CP et al (2013) Peripheral leptin regulates bone formation. J Bone Miner Res 28(1):22–34. doi:10.1002/jbmr.1734
Benkhoff S, Loot AE, Pierson I et al (2012) Leptin potentiates endothelium-dependent relaxation by inducing endothelial expression of neuronal NO synthase. Arterioscler Thromb Vasc Biol 32(7):1605–1612. doi:10.1161/ATVBAHA.112.251140
Percival MD, Ouellet M, Campagnolo C et al (1999) Inhibition of cathepsin K by nitric oxide donors: evidence for the formation of mixed disulfides and a sulfenic acid. Biochemistry 38(41):13574–13583
Jamal SA, Reid LS, Hamilton CJ (2013) The effects of organic nitrates on osteoporosis: a systematic review. Osteoporos Int 24(3):763–770. doi:10.1007/s00198-012-2262-9
Kaur H, Halliwell B (1994) Evidence for nitric oxide-mediated oxidative damage in chronic inflammation. Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett 350(1):9–12
Van’t Hof RJ, Ralston SH (2001) Nitric oxide and bone. Immunology 103(3):255–261
Acknowledgments
The author thanks Ralf P. Brandes for critical reading the manuscript and making helpful suggestions. This work was supported by the Deutsche Forschungsgemeinschaft (SFB815/TP1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schröder, K. NADPH oxidases in bone homeostasis and osteoporosis. Cell. Mol. Life Sci. 72, 25–38 (2015). https://doi.org/10.1007/s00018-014-1712-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1712-2