Summary
Sepsis and sepsis syndrome are significant causes of morbidity and mortality in critically ill surgical patients. Despite technological and therapeutic advances in critical care, sepsis continues to be a pivotal factor in 20 to 50% of deaths in surgical intensive care units. It is clear that alternative approaches to the prevention and/or treatment of sepsis must be found.
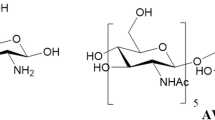
Preclinical data indicate that macrophage activation with (1 →3)-β-D-glucans will ameliorate sequelae associated with Gram-negative septicaemia. We and others have translated these preclinical observations to the clinical setting and have shown that macrophage activation with (1→3)-β-D-glucans will significantly reduce septic morbidity and mortality in trauma and/or high-risk surgical patients. The precise mechanism(s) by which (1→3)-β-D-glucans prevent or ameliorate infections have not been fully elucidated. However, recent data suggest the anti-infective efficacy of (1→3)-β-D-glucans is attributable, in part, to macrophage activation induced by binding of (1→3)-β-D-glucan to a specific receptor followed by modulation of macrophage pro-inflammatory cytokine expression.
This article reviews the anti-infective potential of (1→3)-β-D-glucans in the prevention of sepsis and septic sequelae.
Similar content being viewed by others
References
Bone RC. Gram-negative sepsis. Background, clinical features and intervention. Chest 1991; 100: 802–8
Welbourn CR, Young Y. Endotoxin, septic shock and acute lung injury: neutrophils, macrophages and inflammatory mediators. Br J Surg 1992; 79: 998–1003
Ertel W, Morrison MH, Wang P, et al. The complex pattern of cytokines in sepsis. Association between prostaglandins, cachectin and interleukins. Ann Surg 1991; 214: 141–8
Seatter SC, Bennet T, Li MH, et al. Macrophage endotoxin tolerance: tumor necrosis factor and interleukin-1 regulation by lipopolysaccharide pretreatment. Arch Surg 1994; 129: 1263–70
Ayala A, Perrin MM, Wang P, et al. Sepsis induces an early increased spontaneous release of hepatocellular stimulatory factor (interleukin-6) by Kupffer cells in both endotoxin tolerant and intolerant mice. J Surg Res 1992; 52: 635–41
Ayala A, Perrin MM, Kidala JM, et al. Polymicrobial sepsis selectively activates peritoneal but not alveolar macrophages to release inflammatory mediators (interleukin-1 and -6) and tumor necrosis factor. Circ Shock 1992; 36: 191–9
Natanson C, Hoffman WD, Suffredini AF, et al. Selected treatment strategies for septic shock based on proposed mechanisms of pathogenesis. Ann Intern Med 1994; 120: 771–83
McCloskey RV, Straube RC, Sanders C, et al. Treatment of septic shock with human monoclonal antibody HA-1A. A randomized, double-blind, placebo-controlled trial. CHESS Trial Study Group [see comments]. Ann Intern Med 1994; 121: 1–5
Zanetti G, Glauser MP, Baumgartner JD. Anti-endotoxin antibodies and other inhibitors of endotoxin. New Horizons 1993; 1: 110–9
Fisher Jr CJ, Dhainaut JF, Opal SM, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 1994; 271: 1836–43
Dries DJ, Jurkovick GJ, Maier RV, et al. Effect of interferon gamma on infection-related death in patients with severe injuries: a randomized, double-blind, placebo-controlled trial. Arch Surg 1994; 129: 1031–41
Browder W, Williams D, Pretus H, et al. Beneficial effect of enhanced macrophage function in the trauma patient. Ann Surg 1990; 211: 605–13
Babineau TJ, Marcello P, Swails W, et al. Randomized phase I/II trial of a macrophage-specific immunomodulator (PGG-glucan) in high-risk surgical patients. Ann Surg 1994; 220: 601–9
Babineau TJ, Hackford A, Kenler A, et al. A phase II multicenter, double-blind, randomized, placebo-controlled study of three dosages of an immunomodulator (pgg-glucan) in high-risk surgical patients. Arch Surg 1994; 129: 1204–10
Williams DL, Browder IW, DiLuzio NR. Immunotherapeutic modification of Escherichia coli-induced experimental peritonitis and bacteremia by glucan. Surgery 1983; 93: 448–54
Browder W, Williams D, Sherwood E, et al. Synergistic effect of nonspecific immunostimulation and antibiotics in experimental peritonitis. Surgery 1987; 102: 206–14
Moore JN, Cook JA, Morris DD, et al. Endotoxin-induced procoagulant activity, eicosanoid synthesis, and tumor necrosis factor production by rat peritoneal macrophages: effect of endotoxin tolerance and glucan. Circ Shock 1990; 31: 281–95
Sherwood ER, Williams DL, McNamee RB, et al. Enhancement of interleukin-1 and interleukin-2 production by soluble glucan. Int J Immunopharmacol 1987; 9: 261–7
Rasmussen LT, Seljelid R. Dynamics of blood components and peritoneal fluid during treatment of murine E. coli sepsis with beta-1,3-D-polyglucose derivatives. I. Cells. Scand J Immunol 1990; 32: 321–31
Hoffman OA, Olson EJ, Limper AH. Fungal β-glucans modulate macrophage release of tumor necrosis factor-α in response to bacterial lipopolysaccharide. Immunol Lett 1993; 37: 19–25
Pretus HA, Browder IW, Lucore P, et al. Macrophage activation decreases macrophage prostaglandin E2 release in experimental trauma. J Trauma 1989; 29: 1152–7
Williams DL, McNamee RB, Jones EL, et al. A method for the solubilization of a (1–3)-β-D-glucan isolated from Saccharomyces cerevisiae. Carbohydr Res 1991; 219: 203–13
Marchessault RH, Deslandes Y. Fine structure of (1→3)-D-glucans: curdlan and paramylon. Carbohydr Res 1979; 75: 231–42
Deslandes Y, Marchessault RH, Sarko A. Triple-helical structure of (1→3)-D-glucan. Macromolecules 1980; 13: 1466–71
Bluhm TL, Deslandes Y, Marchessault RH, et al. Solid-state and solution conformation of scleroglucan. Carbohydr Res 1982; 100: 117–30
Williams DL, Sherwood ER, Browder IW, et al. Effect of glucan on neutrophil dynamics and immune function in Escherichia coli peritonitis. J Surg Res 1988; 44: 54–61
Pretus HA, Ensley HE, McNamee RB, et al. Isolation, physicochemical characterization and preclinical efficacy evaluation of soluble scleroglucan. J Pharmacol Exp Ther 1991; 257(1): 500–10
Mansell PWA, Ichinose H, Reed RJ, et al. Macrophage-mediated destruction of human malignant cells in vivo. J Natl Cancer Inst 1975; 54(3): 571–80
Williams DL, Sherwood ER, McNamee RB, et al. Therapeutic efficacy of glucan in a murine model of hepatic metastatic disease. Hepatology 1985; 5: 198–206
Cook JA, Dougherty WJ, Holt TM. Enhanced sensitivity to endotoxin induced by the RE stimulant, glucan. Circ Shock 1980; 7: 225–38
Bowers GJ, Patchen ML, MacVittie TJ, et al. A comparative evaluation of particulate and soluble glucan in an endotoxin model. Int J Immunopharmacol 1986; 8: 313–21
Rasmussen LT, Fandrem J, Seljelid R. Dynamics of blood components and peritoneal fluid during treatment of murine E. coli sepsis with beta-1,3-D-polyglucose derivatives. II. Interleukin 1, tumour necrosis factor, prostaglandin E2, and leukotriene B4. Scand J Immunol 1990; 32: 333–40
Doita M, Rasmussen LT, Seljelid R, et al. Effect of soluble aminated beta-1,3-D-polyglucose on human monocytes: stimulation of cytokine and prostaglandin E2 production but not antigen-presenting function. J Leukoc Biol 1991; 49: 342–51
Williams DL, Pretus HA, McNamee RB, et al. Development of a water-soluble, sulfated (1–3)-β-D-glucan biological response modifier derived from Saccharomyces cerevisiae. Carbohydr Res 1992; 235: 247–57
Williams DL, Pretus HA, McNamee RB, et al. Development, physicochemical characterization and preclinical efficacy evaluation of a water soluble glucan sulfate derived from Saccharomyces cerevisiae. Immunopharmacology 1991; 22: 139–56
Felippe J, Silva M, Maciel FM, et al. Infection prevention in patients with severe multiple trauma with the immunomodulator beta 1–3 polyglucose (glucan). Surg Gynecol Obstet 1993; 177: 383–8
Faist E, Mewes A, Baker CC, et al. Prostaglandin E2 (PGE2) dependent suppression of interleukin alpha (IL-2) production in patients with major trauma. J Trauma 1987; 27: 837–48
Antrum RM, Solomkin JS. Monocyte dysfunction in severe trauma: evidence for the role of C5a in deactivation. Surgery 1986; 100: 29–37
Fife D, Kraus J. Infection as a contributory cause of death in patients hospitalized for motor vehicle trauma. Am J Surg 1988; 155: 278–82
Salmi LR, Williams JI, Waxweiler RJ. Measuring the impact of trauma care on survival: rates of preventable death, effectiveness, and efficacy. J Clin Epidemiol 1990; 43: 399–403
Czop JK, Austen KF. Properties of glycans that activate the human alternative complement pathway and interact with the human monocyte beta-glucan receptor. J Immunol 1985; 135: 3388–93
Czop JK, Austen KF. Generation of leukotrienes by human monocytes upon stimulation of their beta-glucan receptor during phagocytosis. Proc Natl Acad Sci USA 1985; 82: 2751–5
Czop JK, Austen KF. Beta-glucan inhibitable receptor on human monocytes: its identity with the phagocytic receptor for particulate activators of the alternative complement pathway. J Immunol 1985; 134: 2588–93
Goldman R. Induction of a beta-1,3-D-glucan receptor in P388D1 cells treated with retinoic acid or 1,25-dihydroxyvitamin D3. Immunology 1988; 63: 319–24
Czop JK, Kay J. Isolation and characterization of β-glucan receptors on human mononuclear phagocytes. J Exp Med 1991; 173: 1511–20
Leibovici J, Stark Y, Eldar T, et al. Mechanism of the inhibitory effect of levan on experimental tumors. Recent Results Cancer Res 1980; 75: 173–9
Williams JD, Topley N, Alobaidi HM, et al. Activation of human polymorphonuclear leucocytes by particulate zymosan is related to both its major carbohydrate components: glucan and mannan. Immunology 1986; 58: 117–24
Janusz MJ, Austen KF, Czop JK. Isolation of a yeast heptaglucoside for monocyte phagocytic β-glucan receptors. FASEB J 1989; 3: 6421
Christ WJ, Asano O, Robidoux ALC, et al. E5531, a pure endotoxin antagonist of high potency. Science 1995; 268: 80–3
Sherwood ER, Williams DL, Di Luzio NR. Glucan stimulates production of antitumor cytolytic/cytostatic factor(s) by macrophages. J Biol Response Mod 1986; 5: 504–26
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Williams, D.L., Mueller, A. & Browder, W. Glucan-Based Macrophage Stimulators. Clin. Immunother. 5, 392–399 (1996). https://doi.org/10.1007/BF03259335
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03259335