Abstract
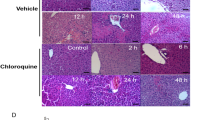
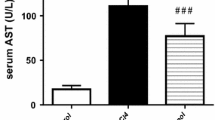
A single administration of a subtoxic dose of CCl4 (100 μl/kg, i.p.) is known to induce hepatocellular regeneration and tissue repair at 6 and 48 h in rats, permitting prompt recovery from the limited liver injury associated with that dose of CCl4. Substantial evidence has accumulated to indicate that the early-phase hepatocellular regeneration and tissue repair are critical for recovery from halomethane hepatotoxicity. The objective of these studies was to test this concept in an experimental framework, wherein a selective ablation of the early-phase cell division should result in prolongation of liver injury followed by recovery. The studies were designed to evaluate the influence of the antimitotic agent colchicine (1 mg/kg, i.p. in saline) on CCl4 toxicity. Colchicine was administered 2 h prior to CCl4 or corn oil injection. Toxicological end points and markers of hepatocellular regeneration were assessed at various time points (2, 6, 12, 24, 48 and 72 h) after the injection of CCl4 to male Sprague-Dawley rats. Hepatocellular injury was assessed through elevations of serum alanine and aspartate aminotransferase and by histopathological examination of the liver. Incorporation of3H-thymidine in hepatocellular nuclear DNA and mitotic index were used as indices of hepatocellular regeneration. Hepatocellular regeneration stimulated by CCl4 at 2–6 h was blocked by colchicine as evidenced by the decreased3H-thymidine incorporation and mitotic index, without any significant effect on the second phase of cell division at 48 h. Ablation of this early phase of tissue repair resulted in prolongation of CCl4 hepatotoxicity. Rats treated with CCl4 alone recovered promptly within 24 h, whereas, colchicine pretreated rats recovered from liver injury after 48 h. Morphometric analysis of hepatocellular necrosis revealed that liver injury at 6 and 12 h after CCl4 was similar in rats regardless of colchicine pretreatment, indicating that prolongation of liver injury was due to delayed liver tissue healing mechanisms. The possibility that prolongation of hepatotoxicity is due to colchicine-induced enhancement of CCl4 metabolism was further investigated in vivo.14CCl4-derived14CO2 exhalation, covalent binding of14CCl4 and14CCl4-derived total radiolabel in the liver and lipid peroxidation were unaltered by colchicine pretreatment. These findings suggest the pivotal importance of the early- as well as the late-phase stimulation of hepatocellular regeneration and tissue healing processes in determining the final outcome of CCl4-induced liver injury.
Similar content being viewed by others
Abbreviations
- ALT:
-
alanine transaminase
- AST:
-
aspartate transaminase
- CLC:
-
colchicine
- CD:
-
chlordecone
- 3H-T:
-
3H-thymidine
- PB:
-
phenobarbital
References
Agarwal AK, Mehendale HM (1983) Potentiation of CCl4 hepatotoxicity and lethality by chlordecone in female rats. Toxicology 26: 231–242
Bell AN, Young RA, Lockard VG, Mehendale HM (1988) Protection of chlordecone-potentiated carbon tetrachloride hepatotoxicity and lethality by partial hepatectomy. Arch Toxicol 61: 392–405
Bruckner JV, Mackenjie WF, Muralidhara S, Luthra R, Kyle GM, Acosta D (1986) Oral toxicity of carbon tetrachloride: acute, subacute and subchronic studies in rats. Fundam Appl Toxicol 6: 16–34
Burton K (1956) A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of DNA. Biochem J 62: 315–323
Butler TC (1961) Reduction of CCl4 in vivo and reduction of CCl4 in vitro by tissue and tissue constituents. J Pharmacol Exp Ther 134: 311–319
Cai Z, Mehendale HM (1990) Lethal effects of CCl4 and its metabolism by Mongolian gerbils pretreated with chlordecone, phenobarbital or mirex. Toxicol Appl Pharmacol 104: 511–520
Cai Z, Mehendale HM (1991 a) Hepatotoxicity and lethality of halomethanes in Mongolian gerbils pretreated with chlordecone, phenobarbital and mirex. Arch Toxicol 65: 204–212
Cai Z, Mehendale HM (1991 b) Prestimulation of hepatocellular regeneration by partial hepatectomy decreases toxicity of CCl4 in gerbils. Biochem Pharmacol 42: 633–644
Chang LO, Looney WB (1965) A biochemical and autoradiographic study of the in vitro utilization of tritiated thymidine in regenerating rat liver. Cancer Res 25: 1817–1822
Chaveau J, Moule Y, Ruiller CH (1956) Isolation of pure unaltered liver nuclei, morphology and biochemical composition. Exp Cell Res 11: 317–321
Cheeseman KH, Albano EF, Tomasi A, Slater TF (1985) Biochemical studies on the metabolic action of halogenated alkanes. Environ Health Perspect 64: 85–101
Curtis LR, Mehendale HM (1979) The effect on kepone pretreatment of biliary excretion of xenobiotic in the male rat. Toxicol Appl Pharmacol 47: 295–303
Curtis LR, Mehendale HM (1980) Specificity of chlordecone induced potentiation of carbon tetrachloride hepatotoxicity. Drug Metab Dispos 8: 23–27
Curtis LR, Williams WL, Mehendale HM (1979) Potentiation of hepatotoxicity of carbon tetrachloride following pre-exposure to chlordecone (Kepone) in the male rat. Toxicol Appl Pharmacol 51: 283–293
Higgins GM, Anderson RM (1931) Experimental pathology of the liver. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol 12: 156–202
Hunter AL, Klaassen CD (1975) Biliary excretion of colchicine. J Pharmacol Exp Ther 192: 605–617
Kawahara H, Marcaue N, French W (1989) Effect of agents which rearrange the cytoskeleton in vitro on the structure and function of hepatocyte canaliculi. Lab Invest 60: 692–704
Kirshner MW (1978) Microtubule assembly and nucleation. Int Rev Cytol 54: 1–65
Klingensmith JS, Mehendale HM (1982) Potentiation of CCl4 lethality by chlordecone. Toxicol Lett 11: 149–154
Klingensmith JS, Mehendale HM (1983) Hepatic microsomal metabolism of CCl4 after pretreatment with chlordecone, mirex or phenobarbital in male rats. Drug Metab Dispos 11: 329–334
Klingensmith JS, Lockard VG, Mehendale HM (1983) Acute hepatotoxicity and lethality of CCl4 in chlordecone pretreated rats. Exp Mol Pathol 39: 1–10
Kodavanti PRS, Joshi UM, Mehendale HM (1989a) Chlordecone-potentiated carbon tetrachloride hepatotoxicity in partially hepatectomized rats — a histomorphometric study. J Appl Toxicol 9: 367–375
Kodavanti PRS, Joshi UM, Young RA, Bell AN, Mehendale HM (1989 b) Role of hepatocellular regeneration in chlordecone potentiated hepatotoxicity of carbon tetrachloride. Arch Toxicol 63: 367–375
Kodavanti PRS, Joshi UM, Young RA, Meydrech EF, Mehendale HM (1989 c) Protection of hepatotoxic and lethal effects of CCl4 by partial hepatectomy. Toxicol Pathol 17: 494–505
Kodavanti PRS, Kodavanti UP, Mehendale HM (1991) Carbon tetrachloride-induced alterations of hepatic calmodulin and free calcium levels in rats pretreated with chlordecone Hepatology 13: 230–238
Leevy CM, Hollister RM, Schmid R, MacDonald RA, Davidson CS (1959) Liver regeneration in experimental CCl4 intoxication. Proc Soc Exp Biol Med 102: 672–675
Lockard VG, O'Neal RM, Mehendale HM (1983 a) Chlordecone-induced potentiation of carbon tetrachloride hepatotoxicity: a light and electron microscopic study. Exp Mol Pathol 39: 230–245
Lockard VG, O'Neal RM, Mehendale HM (1983 b) Chlordecone-induced potentiation of carbon tetrachloride hepatotoxicity: a morphometric and biochemical study. Exp Mol Pathol 39: 246–255
Manfredi JJ, Horwitz SB (1984) An antimitotic agent with a new mechanism of action. Pharm Ther 25: 83–125
Mehendale HM (1984) Potentiation of halomethane hepatotoxicity. chlordecone and carbon tetrachloride. Fundam Appl Toxicol 4: 295–308
Mehendale HM (1990) Potentiation of halomethane hepatotoxicity by chlordecone: a hypothesis for the mechanism. Med Hypoth 33: 289–299
Mehendale HM (1991) Commentary: role of hepatocellular regeneration and hepatocellular healing in the final outcome of liver injury. A two-stage model of toxicity. Biochem Pharmacol 42: 1155–1162
Mehendale HM, Klingensmith JS (1988) In vivo metabolism of CCl4 by rats pretreated with chlordecone, mirex or phenobarbital. Toxicol Appl Pharmacol 93: 247–256
Nakata R, Tsukamoto I, Miyoshi M, Kojos S (1985) Liver regeneration after carbon tetrachloride intoxication in the rat. Biochem Pharmacol 34: 586–588
Omura T, Sato R (1964) The carbon monoxide binging pigment of liver microsomes. Evidence for its hemoprotein nature. J Biol Chem 239: 2370–2378
Paul BP, Rubenstein D (1963) Metabolism of carbon tetrachloride and chloroform by the rat. J Pharmacol Exp Ther 141: 141–148
Rao VC, Mehendale HM (1991 a) Colchicine antimitosis abolishes CCl4 autoprotection. Toxicol Pathol 19: 597–606
Rao VC, Mehendale HM (1991 b) Effect of colchicine on hepatobiliary function in CCl4 treated rats. Biochem Pharmacol 42: 2323–2332
Recknagel RO, Glende EA Jr (1977) Lipid peroxidation: a specific form of cellular injury. In: Lee DHK (ed) Handbook of physiology, Section 9. Williams & Wilkins, Baltimore, MD, pp 591–601
Reddy JK, Chiga M, Svoboda D (1969) Initiation of the division cycle of rat hepatocytes following a single injection of thrioacetamide. Lab Invest 20: 405–411
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum oxaloacetic acid glutamic pyruvic transaminase. Am J Clin Pathol 28: 53–56
Reynolds ES, Moslen MT, Treinen RJ (1981) Carbon tetrachloride freeradical hepatotoxin. In: Rodgers MAT, Powers EL (eds) Oxygen and oxy-radicals in chemistry and biology. Academic Press, New York, pp 169–175
Seawright AA, McLean AEM (1967) The effect of diet on CCl4 metabolism. Biochem J 105: 1055–1060
Smuckler EA (1976) Structural and functional changes in acute liver injury. Environ Health Perspect 15: 13–25
Soni MG, Mehendale HM (1991 a) Protection from chlordeconeamplified carbon tetrachloride toxicity by cyanidanol: biochemical studies. Toxicol Appl Pharmacol 108: 46–57
Soni MG, Mehendale HM (1991 b) Protection from chlordeconeamplified carbon tetrachloride toxicity by cyanidanol. Regeneration studies. Toxicol Appl Pharmacol 108: 58–66
Soni MG, Mehendale HM (1991 c) Hepatic regeneration as a possible mechanism for the hepatoprotective action of cyanidanol. Int J Biochem 23: 1369–1372
Thakore KN, Mehendale HM (1991) Role of hepatocellular regeneration in CCl4 autoprotection. Toxicol Pathol 19: 47–58
Tsukamoto I, Kojo S (1989) Effect of colchicine and vincristine on DNA synthesis in regenerating liver. Biochim Biophys Acta 1009: 191–193
Weibel ER, Staubli W, Guagi HR, Hess FA (1969) Correlated morphometric and biochemical studies on the liver cell. J Cell Biol 42: 68–91
Young RA, Mehendale HM (1989) Carbon tetrachloride metabolism in partially hepatectomized and sham-operated rats preexposed to chlordecone. J Biochem Toxicol 4: 211–219
Author information
Authors and Affiliations
Additional information
A preliminary report of these findings was presented at the ASPET 1990 meeting in Milwaukee, WI [Pharmacologist (1990) 32, 272].
Rights and permissions
About this article
Cite this article
Rao, V.C., Mehendale, H.M. Effect of antimitotic agent colchicine on carbon tetrachloride toxicity. Arch Toxicol 67, 392–400 (1993). https://doi.org/10.1007/BF01977400
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01977400