Abstract
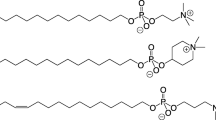
TheN 4-alkylcytosine arabinoside derivativeN 4-octadecyl-AraC (AraC-Ocd, NOAC) and the (1-octadecylglycero-3-phospho)-AraC (Ocd-GroP-AraC, OPA) conjugate are new lipophilic derivatives of the cytostatic drug 1-β-d-arabinofuranosylcytosine (AraC) that produce high antileukemic effects in the L1210 murine leukemia model when administered orally or parenterally as liposomal formulations. Between 83% and 100% of the treated animals were cured after five consecutive daily oral drug applications with a total dose of 1 mmol/kg AraC-Ocd or Ocd-GroP-AraC. Corresponding results were obtained after parenteral therapy on days 2 and 6 after tumor inoculation with five- to ten-fold lower concentrations of these two compounds. A comparable cytotoxic activity was found with the orally active AraC-5′-(n-stearyl phosphate). However, because of its strong hemolytic toxicity this derivative cannot be used for parenteral therapy. Another AraC conjugate, which was modified with two long-chain hydrocarbons, the (1-octadecylglycero-3-phospho)-N 4-hexadecyl-AraC was, probably because of poor oral bioavailability, only active when applied parenterally. The new lipophilic AraC derivatives AraC-Ocd and Ocd-GroP-AraC are compounds with a high potential for the oral treatment of leukemias and possibly also of solid tumors.
Similar content being viewed by others
Abbreviations
- AraC :
-
1-β-d-arabinofuranosylcytosine
- AraC-Ocd :
-
N 4-octadecyl-1-β-d-arabinofuranosylcytosine
- Ocd-GroP-AraC :
-
5′-O-(1-octadecyl-rac-glycero-3-phospho)-1-β-d-arabinofuranosylcytosine
- OcdP-AraC :
-
1-β-d-arabinofuranosylcytosine-5′-(n-stearylphosphate)
- Ocd-GroP-AraC-Hxd :
-
5′-O-(1-octadecyl-rac-glycero-3-phospho)-N 4-hexadecyl-1-β-d-arabinofuranosylcytosine
References
Chabner BA (1990) Cytidine analogues In: Chabner BA, Collins JM (eds), Cancer Chemotherapy, Principles and Practice, Lippincott, Philadelphia, pp 154–179
Ho DHW, Frei E (1971) Clinical pharmacology of 1-β-d-arabinofuranosylcytosine. Clin Pharmacol Ther 12:944–954
Horber DH, Ottiger C, Schott H, Schwendener RA (1995a) Pharmacokinetic properties and interactions with blood components ofN 4-hexadecyl-1-β-d-arabinofuranosylcytosine (NHAC) incorporated into liposomes. J Pharm Pharmacol 47:282–288
Horber DH, Schott H, Schwendener, RA (1995b) Cellular pharmacology of a liposomal preparation ofN 4-hexadecyl-1-β-d-arabinofuranosylcytosine, a lipophilic derivative of 1-β-d-arabinofuranosylcytosine. Br J Cancer 71:957–962
Horber DH, von Ballmoos P, Schott H, Schwendener RA (1995c) Cell cycle dependent cytotoxicity and induction of apoptosis by liposomalN 4-hexadecyl-1-β-d-arabinofuranosylcytosine. Br J Cancer 72:1067–1073
Horber DH, Schott H, Schwendener RA (1995d) Cellular pharmacology ofN 4-hexadecyl-1-β-d-arabinofuranosylcytosine (NHAC) in the human leukemic cell lines K-562 and U-937. Cancer Chemother Pharmacol 36:483–492
Kodama K, Morozumi M, Saitoh K, Kuninaka A, Yoshino H, Saneyoshi M (1989) Antitumor activity and pharmacology of 1-β-d-arabinofuranosylcytosine-5′-stearylphosphate: an orally active derivative 1-β-d-arabinofuranosylcytosine. Jpn J Cancer Res 80:679–685
Koga K, Iizuka E, Sato A, Ekimoto H, Okada M (1995) Characteristic antitumor activity of cytarabine ocfosfate against human colorectal adenocarcinoma xenografts in nude mice. Cancer Chemother Pharmacol 36:459–462
Ohno R, Hirano M, Yamagata K, Ohara K, Shirakawa S, Hiroty Y, Kobayashi M, Yoshikawa S, Mitomo Y, Iikeda Y and the Tokai Blood Cancer Study group (1986) Treatment of leukemia and myelodysplastic syndromes with orally administeredN 4-palmitoyl-1-β-d-arabino-furanosylcytosine. Cancer Chemother Pharmacol 17:161–164
Ohno R, Tatsumi N, Hirano M, Imai K, Mizoguchi H, Nakamura T, Kosaka M and 10 other authors (1991) Treatment of myelodysplastic syndromes with orally administered 1-β-d-arabinofuranosylcytosine-5′-stearylphosphate. Oncology 48:451–455
Rosowsky A, Kim S-H, Wick MM (1982) Lipophilic 5′-(alkylphosphate) esters of 1-β-d-arabinofuranosylcytosine and itsN 4-acyl and 2,2′-anhydro-3′-O-acyl derivatives as potential prodrugs. J Med Chem 25:171–178
Rubas W, Supersaxo A, Weder HG, Hartmann HR, Hengartner H, Schott H, Schwendener RA (1986) Treatment of murine L1210 leukemia and melanoma B16 with lipophilic cytosine arabinoside prodrugs incorporated into unilamellar liposomes. Int J Cancer 37:149–154
Schleyer E, Braess J, Ramsauer B, Unterhalt M, Kaufmann C, Wilde S, Schüssler M, Hiddemann W (1995) Pharmacokinetics of Ara-CMP-stearate (YNK01): phase I study of the oral Ara-C derivative. Leukemia 9:1085–1090
Schott H, Schwendener RA (1996) Synthesis and structure-activity studies of liposomal phospholipid-N 4-palmitoyl- andN 4-hexadecyl-1-β-d-arabinofuranosylcytosine conjugates on L1210 leukemia. Anti-cancer Drug Design (in press).
Schott H, Häussler MP, Schwendener RA (1994) Synthese von 4-Alkylcytosinnucleosiden und deren cytostatische Wirkung im L1210 Leukämiemodell der Maus. Liebigs Ann Chem 465–470
Schwendener RA, Schott H (1992) Treatment of L1210 murine leukemia with liposome-incorporatedN 4-hexadecyl-1-β-d-arabinofuranosylcytosine. Int J Cancer 51:466–469
Schwendener RA, Horber DH, Ottiger C, Schott H (1995) Preclinical properties ofN 4-hexadecyl- andN 4-octadecyl-1-β-d-arabinofuranosylcytosine in liposomal preparations. J Liposome Res 5:27–47
Schwendener RA, Horber DH, Odermatt B, Schott H (1996) Antitumor activity and pharmacological properties of orally administered lipophilicN 4-alkyl derivatives of 1-β-d-arabinofuranosylcytosine (Ara-C). J Cancer Res Clin Oncol 122:102–108
Ueda T, Kamiya K, Urasaki Y, Wataya S, Kawai Y, Tsutani H, Sugiyama M, Nakamura T (1994) Clinical pharmacology of 1-β-d-arabinofuranosylcytosine-5′-stearylphosphate, an orally administered long-acting derivative of low-dose 1-β-d-arabinofuranosylcytosine. Cancer Res 54:109–113
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwendener, R.A., Schott, H. Lipophilic 1-β-d-arabinofuranosyl cytosine derivatives in liposomal formulations for oral and parenteral antileukemic therapy in the murine L1210 leukemia model. J Cancer Res Clin Oncol 122, 723–726 (1996). https://doi.org/10.1007/BF01209119
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01209119