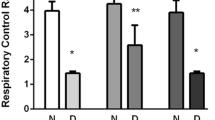
Abstract
The main objective of this study was to determine whether uncontrolled hyperglycemia, as a consequence of diabetes, altered the metabolism of acetylcholine (ACh) in rat brain. To accomplish this, rats received injections of streptozotocin (STZ, 60 mg/kg, i.v.) or vehicle, and were maintained for up to 7 weeks after the injections. Various indices of ACh metabolism were determined in striatum and hippocampus, two brain regions densely innervated by cholinergic neurons. STZ induced diabetes in 96% of the rats injected, as evidenced by glucose spillage into the urine within 48 hours. Serum glucose levels increased to 326% of control values by 1 week and remained at this level for the duration of the study. The steady-state concentrations of ACh and choline, determined in brain tissue from animals killed by head-focused microwave irradiation, did not differ between the control and STZ-injected groups. However, the synthesis and release of neurotransmitter by striatal slices, measured in vitro, decreased in a time-dependent manner. Although the basal release of ACh was unaltered at 1 week, neurotransmitter release decreased significantly by 21% at 5 weeks and by 26% at 7 weeks. The release of ACh evoked by incubation with 35 mM KCl was inhibited significantly by 20% at all time points studied. ACh synthesis by slices incubated under basal conditions decreased by 13% and 27% at 5- and 7-weeks, respectively, the latter significantly less than controls. Synthesis by striatal slices incubated with 35 mM KCl was inhibited by 17% at 7 weeks. Although the synthesis and release of ACh by hippocampal slices from diabetic animals tended to be less than controls, these alterations were not statistically significant. Investigations into the mechanism(s) mediating the deficit in ACh synthesis exhibited by striatal slices indicated that it did not involve alterations in precursor choline availability, nor could it be attributed to alterations in the activities of the synthetic or hydrolytic enzymes choline acetyltransferase or acetylcholinesterase; rather, the decreased turnover of ACh may be secondary to other STZ-induced, hyperglycemia-mediated neurochemical alterations.
Similar content being viewed by others
References
Saller, C. F. 1984. Dopaminergic activity is reduced in diabetic rats. Neurosci. Lett. 49:301–306.
Kwok, R. P. S., and Juorio, A. V. 1986. Concentration of striatal tyramine and dopamine metabolism in diabetic rats and effect of insulin administration. Neuroendocrinology 43:590–596.
Crandall, E. A., Gillis, M. A., and Fernstrom, J. D. 1981. Reduction in brain serotonin synthesis rate in streptozotocin-diabetic rats. Endocrinology 109:310–312.
Trulson, M. E., Jacoby, J. H., and MacKenzie, R. G. 1986. Streptozotocin-induced diabetes reduces brain serotonin synthesis in rats. J. Neurochem. 46:1068–1072.
Bitar, M., Koulu, M., Rapoport, S. I., and Linnoila, M. 1985. Diabetes-induced alteration in brain monoamine metabolism in rats. J. Pharmacol. Exp. Ther. 236:432–437.
Chu, P. C., Lin, M. T., Shian, L. R., and Leu, S. Y. 1986. Alterations in physiologic functions and in brain monoamine content in streptozotocin-diabetic rats. Diabetes 35:481–484.
Lackovic, Z., Salkovic, M., Kuci, S., and Relja, M. 1990. Effect of long-lasting diabetes mellitus on rat and human brain monoamines. J. Neurochem. 54:143–147.
Perlmuter, L. C., Hakami, M. K., Hodgson-Harrington, C., Ginsberg, J., Katz, J., Singer, D. E., and Nathan, D. M. 1984. Decreased cognitive function in aging non-insulin-dependent diabetic patients. Am. J. Med. 77:1043–1048.
Surridge, D. H. C., Williams Erdahl, D. L., Lawson, J. S., Donald, M. W., Monga, T. N., Bird, C. E., and Letemendia, F. J. J. 1984. Psychiatric Aspects of Diabetes Mellitus. Br. J. Psychiatry 145:269–276.
Ryan, C., Vega, A., and Drash, A. 1985. Cognitive deficits in adolescents who developed diabetes early in life. Pediatrics 75:921–927.
Bartus, R. T., Dean, R. L. III, Beer B., and Lippa A. S. 1982. The cholinergic hypothesis of geriatric memory dysfunction. Science 217:408–417.
Davis, K. L., Mohs, R. C., Rosen, W. G., Greenwald, B. S., Levy, M. I., and Horvath, T. B. 1983. Memory enhancement with oral physostigmine in Alzheimer's disease. N. Engl. J. Med. 308:721–723.
Mooradian, A. D. 1987. Blood-brain barrier choline transport is reduced in diabetic rats. Diabetes 36:1094–1097.
Stavinoha, W. B., and Weintraub, S. T. 1974. Choline content of rat brain. Science 183:964–965.
Schmidt, D. E. 1976. Regional levels of choline and acetylcholine in rat brain following head focused microwave sacrifice: Effect of (+)-amphetamine and (±)-parachloroamphetamine. Neuropharmacol. 15:77–84.
Schmidt, D. E., and Speth, R. C. 1975. Simultaneous analysis of choline and acetylcholine levels in rat brain by pyrolysis gas chromatography. Anal. Biochem. 67:353–357.
Flynn, C. J., and Wecker, L. 1986. Elevated choline levels in brain: a non-cholinergic component of organophosphate toxicity. Biochem. Pharmacol. 35:3115–3121.
Fonnum, F. 1969. Isolation of choline esters from aqueous solutions by extraction with sodium tetraphenylboron in organic solvents. Biochem. J. 113:291–298.
Goldberg, A. M., and McCaman, R. E. 1973. The determination of picomole amounts of acetylcholine in mammalian brain. J. Neurochem. 20:1–8.
Doležal, V., and Wecker, L. 1990. Muscarinic receptor blockade increases basal acetylcholine release from striatal slices. J. Pharmacol. Exp. Ther. 252:739–743.
Yamamura, H. I., and Snyder, S. H. 1972. Choline: high-affinity uptake by rat brain synaptosomes. Science 178:626–628.
Haga, T., and Noda, H. 1973. Choline uptake systems of rat brain synaptosomes. Biochim. Biophys. Acta. 291:564–575.
Fonnum, F. 1969. Radiochemical micro assays for the determination of choline acetyltransferase and acetylcholinesterase activities. Biochem. J. 115:465–472.
Tuĉek, S. 1978. Acetylcholine Synthesis in Neurons. London, Chapman and Hall, p. 22–29.
Ellman, G. L., Courtney, K. D., Andres, V., Jr. and Featherstone, R. M. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7:88–95.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275.
Schuberth, J., Sparf, B., and Sundwall, A. 1969. A technique for the study of acetylcholine turnover in mouse brain in vivo. J. Neurochem. 16:695–700.
Schuberth, J., Sparf, B., and Sundwall, A. 1970. On the turnover of acetylcholine in nerve endings of mouse brain in vivo. J. Neurochem. 17:461–468.
Ruderman, N. B., Ross, P. S., Berger, M., and Goodman, M. N. 1974. Regulation of glucose and ketone-body metabolism in brain of anaesthetized rats. Biochem. J. 138:1–10.
Blusztajn, J. K., Liscovitch, M., and Richardson, U. I. 1987. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Proc. Natl. Acad. Sci. 84:5474–5477.
Dyer, J. R., and Greenwood, C. E. 1988. Evidence for altered methionine methyl-group utilization in the diabetic rat's brain. Neurochem. Res. 13:517–523.
Wahba, Z. Z., and Soliman, K. F. A. 1988. Effect of diabetes on the enzymes of the cholinergic system of the rat brain. Experientia 44:742–746.
Das, P. K., Bray, G. M., Aguayo, A. J., and Rasminsky, M. 1976. Diminished ouabain-sensitive, sodium-potassium ATPase activity in sciatic nerves of rats with streptozotocin-induced diabetes. Exp. Neurol. 53:285–288.
Cogan, D. G., Kinoshita, J. H., Kador, P. F., Robinson, W. G., Datilis, M. B., Cobo, L. M., and Kupfer, C. 1984. Aldose reductase and complications of diabetes. Ann. Intern. Med. 101:82–91.
Greene, D. A., and Lattimer, S. A. 1984. Action of sorbinil in diabetic peripheral nerve: Relationship of polyol (sorbitol) path-way inhibition to a myo-inositol-mediated defect in sodium-potassium ATPase activity. Diabetes 33:712–716.
Greene, D. A., and Lattimer, S. A. 1986. Protein kinase C agonists acutely normalize decreased ouabain-inhibitable respiration in diabetic rabbit nerve: Implications for (Na, K)-ATPase regulation and diabetic complications. Diabetes 35:242–245.
Greene, D. A., Lattimer, S. A., and Sima, A. A. F. 1987. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. New Engl. J. Med. 316:599–606.
Schmidt, R. E., Plurad, S. B., Sherman, W. R., Williamson, J. R., and Tilton, R. G. 1989. Effects of aldose reductase inhibitor sorbinol on neuronal dystrophy and levels of myo-inositol and sorbitol in sympathetic autonomic ganglia of streptozotocin-induced diabetic rats. Diabetes 38:569–579.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Welsh, B., Wecker, L. Effects of streptozotocin-induced diabetes on acetylcholine metabolism in rat brain. Neurochem Res 16, 453–460 (1991). https://doi.org/10.1007/BF00965566
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00965566