Abstract
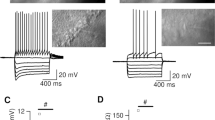
Adenosine added to the perfusion fluid of rat hippocampal slices at 10 μmol · l−1 enhanced long lasting afterhyperpolarizations after single action potentials, bursts of action potentials or calcium spikes. Accommodation of firing during a depolarizing pulse was potentiated. An increase in calcium dependent potassium conductance is likely to mediate these effects. Adenosine at 50 μmol·l−1 induced a hyperpolarization accompanied by a reduction in input resistance. The hyperpolarization could be reversed at −85 mV. In TTX and TTX-barium treated slices the amplitude of the slow spike was decreased. This may result from a shunting of inward current in the dendrites due to an adenosine induced increase in potassium conductance. It is suggested that adenosine reduces pre- and postsynaptic exicatory signals principally by enhancing one or more potassium conductances. This effect is a powerful means for modulation of neuronal excitability and synaptic efficacy and can explain the antiepileptic activity of adenosine.
Similar content being viewed by others
References
Alger BE, Nicoll RA (1980) Epileptiform burst after-hyperpolarization: calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science 210:1122–1124
Armstrong CM, Swenson RP, Taylor SR (1982) Block of squid axon K channels by internally and externally applied barium ions. J Gen Physiol 80:663–682
Benardo LS, Prince DA (1982) Dopamine modulates a Ca2+-activated potassium conductance in mamalian hippocampal pyramidal cells. Nature 297:76–79
Branisteanu DD, Haulica ID, Proca B, Nhue BG (1979) Adenosine effects upon transmitter release parameters in the Mg2+ paralyzed neuro-muscular junction of the frog. Naunyn-Schmiedebergs Arch Pharmacol 308:273–279
Brown DA, Griffith WH (1983) Calcium-activated outward current in voltage-clamped hippocampal neurones of the guinea-pig. J Physiol (Lond) 337:287–301
Burnstock G (1972) Purinergic nerves. Pharmacol Rev 24:509–581
Connor JA (1979) Calcium current in molluscan neurones: measurement under conditions which maximize its visibility. J Physiol (Lond) 286:41–60
Constanti A, Adams PR, Brown DA (1981) Why do barium ions imitate acetylcholine? Brain Res 206:244–250
Dunwiddie TV (1984) Interactions between the effects of adenosine and calcium on synaptic responses in rat hippo-campus in vitro. J Physiol (Lond) 350:545–559
Dunwiddie TV, Hoffer B (1980) Adenine nucleotides and synaptic transmission in the in vitro hippocampus. Br J Pharmacol 69:59–68
Fredholm BB, Hedqvist P (1980) Modulation of neurotransmission by purine nucleosides and nucleotides. Biochem Pharmacol 29:1635–1643
Ginsborg BL, Hirst GDS (1972) The effect, of adenosine on the release of the transmitter from phrenic nerve of the rat. J Physiol (Lond) 224:629–645
Goodman RR, Snyder SH (1982) Autoradiographic localization of adenosine receptors in rat brain using [3H]cyclohexyladenosine. J Neurosci 2:1230–1241
Goodman RR, Kuhar MJ, Hester L, Snyder SH (1983) Adenosine receptors: autoradiographic evidence for their location on axon terminals of excitatory neurons. Science 220:967–968
Gustafsson B, Wigström H (1981) Evidence for two types of afterhyperpolarization in CA1 pyramidal cells in the hippocampus. Brain Res 206:462–468
Haas HL (1984) Histamine potentiates neuronal excitation by blocking a calcium dependent potassium conductance. Agents Actions 14:534–537
Haas HL, Jefferys JGR (1984) Low-calcium field burst discharges of CA1 pyramidal neurones in rat hippocampal slices. J Physiol (Lond) 354:185–201
Haas HL, Konnerth A (1983) Histamine and noradrenaline decrease calcium-activated potassium conductance in hippocampal pyramidal cells. Nature 302:432–434
Haas HL, Rose G (1984) The role of inhibitory mechanisms in hippocampal long term potentiation. Neurosci. Lett 47:301–306
Haas HL, Schärer B, Vosmansky M (1979) A simple perfusion chamber for the study of nervous tissue slices in vitro. J Neurosci Methods 1:323–325
Haas HL, Jefferys JGR, Slater NT, Carpenter DO (1984) Modulation of low calcium induced field bursts in the hippocampus by monoamines and cholinomimetics. Pflügers Arch 400:28–33
Hablitz JJ (1981) Altered burst responses in hippocampal CA3 neurones injected with EGTA. Exp Brain Res 42:483–485
Hagiwara S, Byerly L (1981) Calcium channel. A Rev Neurosci 4:69–125
Halliwell JV, Adams PR (1982) Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res 250:71–92
Henon BK, McAfee DA (1983) The ionic basis of adenosine receptor actions on post-ganglionic neurones in the rat. J Physiol (Lond) 336:607–620
Hood TW, Siegfried J, Haas HL (1983) Analysis of carbamazepine actions in hippocampal slices of the rat. Cell Mol Neurobiol 3:213–222
Hotson JR, Prince DA (1980) A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurones. J Neurophysiol 43:409–419
Johnston D, Hablitz JJ, Wilson WA (1980) Voltage clamp discloses slow inward current in hippocampal burst-firing neurones. Nature 286:391–393
Kuroda Y (1978) Physiological roles of adenosine derivatives which are released during neurotransmission in mammalian brain. J Physiol (Paris) 74:463–470
Madison DV, Nicoll RA (1982) Noradrenaline blocks accommodation of pyramidal cell discharge in the hippocampus. Nature 299:636–638
Nicoll RA, Alger BE (1981) Synaptic excitation may activate a calcium-dependent potassium conductance in hippocampal pyramidal cells. Science 212:957–959
Okada Y, Ozawa S (1980) Inhibitory action of adenosine on synaptic transmission in the hippocampus of the guinea pig in vitro. Eur J Pharmacol 68:483–492
Phillis JW, Edstrom JP, Kostopoulos GK, Kirkpatrick JR (1979) Effects of adenosine and adenine nucleotides on synaptic transmission in the cerebral cortex. Can J Physiol Pharmacol 57:1289–1312
Proctor WR, Dunwiddie TV (1983) Adenosine inhibits calcium spikes in hippocampal pyramidal neurones in vitro. Neurosci Lett 35:197–201
Reddington M, Lee KS, Schubert P (1982) An A1-adenosine receptor, characterized by [3H]cyclohexyladenosine binding, mediates the depression of evoked potentials in a rat hippocampal slice preparation. Neurosci Lett 28:275–279
Rubio R, Berne RM, Winn HR (1978) Production, metabolism and possible functions in brain tissue in situ. Ciba Found Symp 56. Elsevier, Amsterdam, pp 355–378
Scholfield CN (1978) Depression of evoked potentials in brain slices by adenosine compounds. Br J Pharmacol 63:239
Schubert P, Mitzdorf U (1979) Analysis and quantitative evaluation of the depressive effect of adenosine on evoked potentials in hippocampal slices. Brain Res 172:186–190
Schwartzkroin PA, Prince DA (1980) Effects of TEA on hippocampal neurons. Brain Res 185:169–181
Schwartzkroin PA, Slawsky M (1977) Probable calcium spikes in hippocampal neurons. Brain Res 135:157–161
Schwartzkroin PA, Stafström CE (1980) Effects of EGTA on the calcium-activated afterhyperpolarization in hippocampal CA3 pyramidal cells. Science 210:1125–1127
Segal M (1982) Intracellular analysis of a postsynaptic action of adenosine in the rat hippocampus. Eur J Pharmacol 79:193–199
Siggins GR, Schubert P (1981) Adenosine depression of hippocampal neurons in vitro: an intracellular study of dose-dependent actions on synaptic and membrane potentials. Neurosci Lett 23:55–60
Wong RKS, Prince DA (1981) Afterpotential generation in hippocampal pyramidal cells. J Neurophysiol 45:86–97
Wong RKS, Prince DA, Basbaum AI (1979) Intradendritic recordings from hippocampal neurons. Proc Natl Acad Sci USA 76:986–990
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Haas, H.L., Greene, R.W. Adenosine enhances afterhyperpolarization and accommodation in hippocampal pyramidal cells. Pflugers Arch. 402, 244–247 (1984). https://doi.org/10.1007/BF00585506
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00585506