Abstract
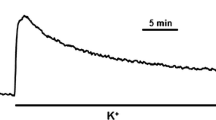
The effects of okadaic acid, a phosphoprotein phosphatase inhibitor, on the contractile response and on myosin light chain phosphorylation were studied in intact lamb tracheal smooth muscle. The effects of okadaic acid were compared to the response to the same fibers stimulated with 1 μM methacholine, a concentration that induces 90% of maximal force. Okadaic acid (50 μM) produced a slow but maximal contraction that was accompanied by an increase in phosphorylation of the 20 kDa light chain of myosin. The myosin light chain phosphorylation pattern induced by okadaic acid, however, differed from that induced by methacholine. Ca2+ depletion, N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide (W-7), a calmodulin antagonist and 1-(5-isoquinolinylsulfonyl)-2-methylpiperazine (H-7), a protein kinase C inhibitor, blocked or attenuated methacholine-induced contractions but had no significant effect on force development or myosin light chain phosphorylation induced by okadaic acid. These results suggest that phosphorylation of the 20 kDa light chain of myosin is essential for smooth muscle contraction; they also suggest that okadaic acid either uncovers or activates an apparently Ca2+ and calmodulin-independent protein kinase activity that phosphorylates the 20 kDa light chain of myosin at multiple sites.
Similar content being viewed by others
Abbreviations
- LC20 :
-
the 20 kDa light chain of smooth muscle myosin
- OA:
-
okadaic acid
- DMSO:
-
dimethylsulfoxide
- W-7:
-
N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide
- H-7:
-
1-(5-isoquinolinylsulfonyl)-2-methylpiperazine
- TBS:
-
150 mM NaCl, 20 mM Tris-HCl, pH 7.5
References
Adelstein RS, Klee CB (1981) Purification and characterization of smooth muscle myosin light chain kinase. J Biol Chem 256:7501–7509
Aksoy MO, Murphy RA, Kamm KE (1982) Role of Ca2+ and myosin light chain phosphorylation in regulation of smooth muscle. Am J Physiol 242:C109-C116
Bengur AR, Robinson EA, Appella E, Sellers JR (1987) Sequence of the sites phosphorylated by protein kinase C in the smooth muscle myosin light chain. J Biol Chem 262:7613–7617
Bialojan C, Ruegg JC, Takai A (1988) Effects of okadaic acid on isometric tension and myosin phosphorylation of chemically skinned guinea-pig taenia coli. J Physiol 398:81–95
Cody RP, Smith JK (1987) Hypothesis testing (two groups). In: Cody RP, Smith JK (eds) Applied statistics and the SAS programming language, 2nd edn. North-Holland, New York, pp 92–95
Dabrowska R, Sherry JFM, Aromatorio DK, Hartshorne DJ (1978) Modulator protein as a component of the myosin light chain kinase from chicken gizzard. Biochemistry 17:253–258
de Lanerolle P (19889) Regulation of airway muscle responses. In: Massaro D (ed) Lung biology in health and disease. Dekker, New York Basel, pp 153–189
de Lanerolle P, Nishikawa M (1988) Regulation of embryonic smooth muscle myosin by protein kinase C. J Biol Chem 263:9071–9074
de Lanerolle P, Stull JT (1980) Myosin phosphorylation during contraction and relaxation of tracheal smooth muscle. J Biol Chem 255:9993–10000
de Lanerolle P, Condit JR Jr, Tanenbaum M, Adelstein RS (1982) Myosin phosphorylation, agonist concentration and concentration of tracheal smooth muscle. Nature 298:871–872
Gerthoffer WT (1986) Calcium dependence of myosin phosphorylation and airway smooth muscle contraction and relaxation. Am J Physiol 250:C597-C604
Haeberle JR, Hott JW, Hathaway DR (1985) Regulation of isometric force and isotonic shortening velocity by phosphorylation of the 20,000 dalton myosin light chain of rat uterine smooth muscle. Pflügers Archiv 403:215–219
Haeberle JR, Sutton TA, Trockman BA (1988) Phosphorylation of two sites on smooth muscle myosin. J Biol Chem 263:4424–4429
Hidaka H, Inagaki M, Kawamoto S, Sasaki Y (1984) Isoquinoline-sulfonamines, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry 23:5036–5041
Ikebe M, Hartshorne DJ, Elzinga M (1987) Phosphorylation of the 20,000-dalton light chain of smooth muscle myosin by the calcium-activated, phospholipid-dependent protein kinase. J Biol Chem 262:9569–9573
Itoh T, Kuriyama H, Suzuki H (1983) Differences and similarities in the noradrenaline- and caffeine-induced mechanical responses in the rabbit mesenteric artery. J Physiol 337:609–629
Mayer SE (1980) Neurohumoral transmission and the autonomic nervous system. In: Gilman AG, Goodman L, Gilman A (eds) Goodman and Gilmans the pharmacological basis of therapeutics, 6th edn. Macmillan, London, pp 56–90
Nishikawa M, Hidaka H, Adelstein RS (1983) Phosphorylation of smooth muscle heavy meromyosin by calcium-activated, phospholipid-dependent protein kinase. J Biol Chem 258:14069–14072
Ozaki H, Ishihara H, Kohama K, Nonomura Y, Shibata S, Karaki H (1987) Calcium-independent phosphorylation of smooth muscle myosin light chain by okadaic acid isolated from black sponge. J Pharmacol Exp Ther 243:1167–1173
Sellers JR, Pato MD, Adelstein RS (1981) Reversible phosphorylation of smooth muscle myosin, heavy meromyosin, and platelet myosin. J Biol Chem 256:13137–13142
Shibata S, Ishida Y, Kitano H, Ohizumi Y, Habon J, Tsukitani Y, Kikuchi H (1982) Contractile effects of okadaic acid, a novel ionophore-like substance from black sponge, on isolated smooth muscle under the condition of Ca deficiency. J Pharmacol Exp Ther 233:135–143
Suganuma M, Fujiki H, Suguri H, Yoshizawa S, Hirota M, Nakayasu M, Ojika M, Wakamatsu K, Yamada K, Sugimura T (1988) Okadaic acid: An additional nonphorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc Natl Acad Sci USA 85:1768–1771
Takai A, Bialojan C, Troschka M, Ruegg JC (1987) Smooth muscle myosin phosphatase inhibition and force enhancement by black sponge toxin. FEBS Lett 217:81–84
Tanaka T, Ohmura T, Yamakoda T, Hidaka H (1982) Two types of calcium-dependent protein phosphorylations modulated by calmodulin antagonists. Mol Pharmacol 22:408–412
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Obara, K., Takai, A., Ruegg, J.C. et al. Okadaic acid, a phosphatase inhibitor, produces a Ca2+ and calmodulin-independent contraction of smooth muscle. Pflugers Arch. 414, 134–138 (1989). https://doi.org/10.1007/BF00580954
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00580954