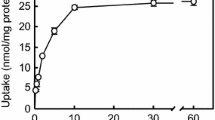
Abstract
In order to evaluate whether N-containing substrates interact with the organic “anion” (p-aminohippurate, PAH) or only with the organic “cation” (N 1-methylnicotinamide, NMeN) transport system or with both, the stop-flow peritubular capillary microperfusion method was applied in the rat kidney in situ and the apparent K i values of several classes or organic substrate against contraluminal NMeN and PAH transport were determined. Organic “anion” and organic “cation” transport are in inverted commas because neither transporter sees the degree of ionization in bulk solution, and they also accept nonionizable substrates [Ullrich KJ, Rumrich G (1992) Pflügers Arch 421:286–288]. Amines must be sufficiently hydrophobic (phenylethylamine, piperidine, piperazine) in order to interact with NMeN transport. Additional Cl, Br, NO2 or other electronegative groups render them inhibitory towards PAH transport also. Such bisubstrate amines were identified as follows: metoclopramide, bromopride, diphenhydramine, bromodiphenhydramine, verapamil, citalopram, ketamine, mefloquine, ipsapirone, buspirone, trazodone, H7 and trifluoperazine. Imidazole analogues interact with both transporters if they bear sufficiently hydrophobic alkyl or aryl groups or electronegative sidegroups. Bisubstrate imidazole analogues are tinidazole, pilocarpine, clonidine, azidoclonidine and cimetidine. Pyridines and thiazoles interact with the NMeN transporter if they have an additional ring-attached NH2 group. Again with an additional Cl, Br, or NO2 group the aminopyridines and aminothiazoles also become inhibitors for the PAH transporter. Amongst the guanidines only substances with several electronegative side-groups such as guanfacine, amiloride, benzylamiloride and ranitidine, interact with both transporters. Amongst the phenylhydrazines only 4-bromophenylhydrazine interacts with the NMeN transporter and 4-nitrophenylhydrazine with both transporters. Quinoline (isoquinoline) and its amino and hydroxy analogues interact with both transporters, their pKa values correlate directly with the affinity to the NMeN transporter and reciprocally with their affinity to the PAH transporter. In experiments with labelled substrates only the sufficiently hydrophilic cimetidine, amiloride and ranitidine show a saturable transport, which can be inhibited by probenecid (apalcillin) and tetraethylammonium in an additive manner. The highly hydrophobic substrates verapamil, citalopram, imipramine, diltiazem and clonidine enter the cell very fast in an unsaturable and uninhibitable manner, apparently in the undissociated form, since N-methyl-4-phenylpyridinium, which — disregarding its ionization — is similarly hydrophobic, shows a transport behaviour similar to that of tetraethylammonium [Ullrich et al. (1991) Pflügers Arch 419:84–92]. Ethidium bromide and dimidium bromide, which have a permanent cationic quaternary nitrogen and two sufficiently electronegative NH2 groups, also interact with both transporters. The data indicate that a molecule qualifies as a bisubstrate if it carries both the essentials for organic anion (PAH) transport: hydrophobicity, sufficient acidity or electron-attracting O, OH, Cl, Br, NO2 groups, plus the essentials for organic cation transport: hydrophobicity, sufficient basicity or electron-donating N-containing groups. The nitrogen atoms in the N-containing molecules quinoline (pK a 4.9), isoquinoline (pK a 5.4) and benzylpyridine (pK a 5.13) are of such low basicity that they apparently can also interact with the PAH transporter. Apparent hydrophobicity (disregarding ionization) determines interaction with the transporters, while real hydrophobicity [log (octanol distribution values)] determines the diffusion through the lipid bilayer of the cell membrane.
Similar content being viewed by others
References
Alvarado F, Brot-Laroche E, L'Herminier M, Murer H, Stange G (1979) The effect of harmaline on intestinal sodium transport and on sodium-dependent d-glucose transport in brushborder membrane vesicles from rabbit jejunum. Pflügers Arch 382: 35–41
Boom SPA, Gribnau FWJ, Russel FGM (1992) Organic cation transport and cationic drug interactions in freshly isolated proximal tubular cells of the rat. J Pharmacol Exp Ther 263: 445–450
Cacini W, Keller MB, Grand VR (1982) Accumulation of cimetidine by kidney cortex slices. J Pharmacol Exp Ther 221: 342–346
Chan YL, Huang KC (1973) Renal excretion of d-tryptophan, 5-hydroxytryptamine, and 5-hydroxyindoleacetic acid in rats. Am J Physiol 224: 140–143
Dantzler WH, Brokl OH (1984) Verapamil and quinidine effects on PAH transport by isolated perfused renal tubules. Am J Physiol 246: F 188-F 200
Despopoulos A (1965) A definition of substrate specificity in renal transport of organic anions. J Theor Biol 8: 163–192
Elsenhans B, Blume R, Lembcke B, Caspary WF (1985) In vitro inhibition of rat small intestinal absorption by lipophilic organic cations. Biochim Biophys Acta 813: 25–32
Frelin C, Vigne P, Barbry P, Lazdunski M (1986) Interaction of guanidinium and guanidinium derivatives with the Na+/H+ exchange system. Eur J Biochem 154: 241–245
Friedrich T, Burckhardt G (1988) Inhibition and labeling of the rat renal Na+/H+ exchanger by an antagonist of muscarinic acetylcholine receptors. Biochem Biophys Res Commun 157: 921–929
Friedrich T, Burckhardt G (1991) Inhibition of the rat renal Na+/H+ exchanger by β-adrenergic antagonists. Biochem Biophys Res Commun 175: 311–317
Fritzsch G, Haase W, Rumrich G, Fasold H, Ullrich KJ (1984) A stopped flow capillary perfusion method to evaluate contraluminal transport parameters of methylsuccinate from interstitium into renal proximal tubular cells. Pflügers Arch 400: 250–256
Fritzsch G, Rumrich G, Ullrich KJ (1989) Anion transport through the contraluminal cell membrane of renal proximal tubule. The influence of hydrophobicity and molecular charge distribution on the inhibitory activity of organic anions. Biochim Biophys Acta 978: 249–256
Gisclon G, Boyd RA, Williams RL, Giacomini KM (1989) The effect of probenecid on the renal elimination of cimetidine. Clin Pharmacol Ther 45: 444–452
Herzfelt CD (1980) Dissoziationskonstanten von Arzneistoffen: Eine Übersichtstabelle. Pharm Ztg 12: 608–614
Inotsume N, Nishimura M, Nakano M, Fujiyama S, Sato T (1990) The inhibitory effect of probenecid on renal excretion of famotidine in young, healthy volunteers. J Clin Pharmacol 30: 50–56
Jeffrey GA, Saenger W (1991) Hydrogen bonding in biological structures. Springer, Berlin Heidelberg New York
Jensen RE, Berndt WO (1988) Epinephrine and norepinephrine enhance p-aminohippurate transport into basolateral membrane vesicles. J Pharmacol Exp Ther 244: 543–549
Kleyman TR, Cragoe EJ Jr (1988) Amiloride and its analogs as tools in the study of ion transport. J Membr Biol 105: 1–21
Koepsell H, Fritzsch G, Korn K, Madrala A (1990) Two substrate sites in the renal Na+-d-glucose cotransporter studied by model analysis of phlorizin binding and d-glucose transport measurements. J Membr Biol 114: 113–132
Kribben A, Wetzels JFM, Wieder ED, Burke TJ, Schrier RW (1993) New technique to assess hypoxia-induced cell injury in individual isolated renal tubules. Kidney Int 43: 464–469
Kulanthaivel P, Leibach FH, Mahesh VB, Cragoe EJ Jr, Gana-pathy V (1990) The Na+-H+ exchanger of the placental brushborder membrane is pharmacologically distinct from that of the renal brush-border membrane. J Biol Chem 265: 1249–1252
Lappe RW, Henry DP, Willis LR (1980) Mechanism of renal tubular secretion of norepinephrine in the rabbit. J Pharmacol Exp Ther 215: 443–449
Lin JH, Los LE, Ulm EH, Duggan DE (1988) Kinetic studies on the competition between famotidine and cimetidine in rats: evidence of multiple renal secretory systems for organic cations. Drag Metab Dispos 16: 52–56
McKinney TD, Myers P, Speeg KV Jr (1981) Cimetidine secretion by rabbit renal tubules in vitro. Am J Physiol 241: F 69-F 76
Miyamoto Y, Balkovetz DF, Ganapathy V, Iwatsubo T, Hanano M, Leibach FH (1988) Effects of phenothiazines on the Na+-H+ exchanger of the brush-border membrane from the proximal small intestine of the rabbit. J Pharmacol Exp Ther 245: 823–828
Møller JV, Sheikh MI (1983) Renal organic anion transport system: pharmacological, physiological, and biochemical aspects. Pharmacol Rev 34: 315–358
Nunnari JM, Repaske MG, Brandon S, Cragoe EJ Jr, Limbird LE (1987) Regulation of porcine brain α 2-adrenergic receptors by Na+, H+ and inhibitors of Na+/H+ exchange. J Biol Chem 262: 12 387–12 392
Ott RJ, Hui AC, Giacomini KM (1990) Mechanisms of interaction between organic anions and the organic cation transporter in renal brush border membrane vesicles. Biochem Pharmacol 40: 659–661
Quebbemann AJ, Rennick BR (1969) Effects of structural modifications of catecholamines on renal tubular transport in the chicken. J Pharmacol Exp Ther 166: 52–62
Raymond GG, Born JL (1986) An updated pK a listing of medicinal compounds. Drag Intell Clin Pharm 20: 683–686
Rennick BR (1981) Renal tubular transport of organic cations. In: Greger R, Lang F, Silbernagl S (eds) Renal transport of organic substances. Springer, Berlin Heidelberg New York, pp 178–188
Roch-Ramel F, Besseghir K, Murer H (1992) Renal excretion and tubular transport of organic anions and cations. In: Windhager EE (ed) Handbook of Physiology, sect 8, vol II. Renal physiology. Oxford Univ. Press, New York, pp 2189–2262
Sewing K, Kaplowitz N (1979) Drugs affecting the pharmakokinetics of 14C-cimetidine in rats. Gastroenterology 76: 1242
Sokol PP (1990) Effect of DQ-2556, a new cephalosporin, on organic ion transport in renal plasma membrane vesicles from the dog, rabbit and rat. J Pharmacol Exp Ther 255: 436–441
Sokol PP, Huiatt KR, Holohan PD, Ross CR (1989) Gentamicin and verapamil compete for a common transport mechanism in renal brush border membrane vesicles. J Pharmacol Exp Ther 251: 937–942
Sperber I (1959) Secretion of organic anions in the formation of urine and bile. Pharmacol Rev 11: 109–134
Suzuki H, Sawada Y, Sugiyama Y, Iga T, Hanano M (1986) Transport of cimetidine by the rat choroid plexus in vitro. J Pharmacol Exp Ther 239: 927–935
Ullrich KJ, Rumrich G (1988) Contraluminal transport systems in the proximal renal tubule involved in secretion of organic anions. Am J Physiol 254: F 453-F 462
Ullrich KJ, Rumrich G (1990) Kidney: microperfusion — double-perfused tubule in situ. Methods Enzymol 191: 98–107
Ullrich KJ, Rumrich G (1992) Renal contraluminal transport systems for organic anions (para-aminohippurate, PAH) and organic cations (N 1-methyl-nicotinamide, NMeN) do not see the degree of substrate ionization. Pflügers Arch 421: 286–288
Ullrich KJ, Rumrich G, Klöss S (1988) Contraluminal paraaminohippurate (PAH) transport in the proximal tubule of the rat kidney. IV. Specifity: mono- and polysubstituted benzene analogs. Pflügers Arch 413: 134–146
Ullrich KJ, Papavassiliou F, David C, Rumrich G, Fritzsch G (1991) Contraluminal transport or organic cations in the proximal tubule of the rat kidney: I. Kinetics of N 1-methylnicotinamide and tetraethylammonium, influence of K+, HCO3 −, pH, inhibition by aliphatic primary, secondary and tertiary amines, and mono- and bisquarternary compounds. Pflügers Arch 419: 84–92
Ullrich KJ, Rumrich G, Papavassiliou F, Hierholzer K (1991) Contraluminal p-aminohippurate transport in the proximal tubule of the rat kidney. VIII. Transport of corticosteroids. Pflügers Arch 418: 371–382
Ullrich KJ, Rumrich G, Papavassiliou F, Klöss S, Fritzsch G (1991) Contraluminal p-aminohippurate transport in the proximal tubule of the rat kidney. VII. Specificity: cyclic nucleotides, eicosanoids. Pflügers Arch 418: 360–370
Ullrich KJ, Rumrich G, Fritzsch G (1992) Substrate specificity of the organic anion and organic cation transport systems in the proximal renal tubule. In: Bamberg E, Passow H (eds) Progress in cell research. Elsevier, Amsterdam 2: 315–321
Ullrich KJ, Rumrich G, Neiteler K, Fritzsch G (1992) Contraluminal transport of organic cations in the proximal tubule of the rat kidney. II. Specificity: anilines, phenylalkylamines (catecholamines), heterocyclic compounds (pyridines, quinolines, acridines). Pflügers Arch 420: 29–38
Ullrich KJ, Rumrich G, David C, Fritzsch G (1993) Bisubstrates: substances that interact with both, renal contraluminal organic anion and organic cation transport systems. II. Zwitterionic substrates: dipeptides, cephalosporins, quinolone-carboxylate gyrase inhibitors, and phosphamide thiazine carboxylates; nonionizable substrates: steroid hormones and cyclophosphamides. Pflügers Arch 425: 300–312
Weiner IM (1973) Transport of weak acids and bases. In: Orloff J, Berliner RW (eds) Handbook of Physiology, sect. 8. Renal physiology. Am. Physiol. Soc, Washington, pp 521–554
Williams PD, Hitchcock MJM, Hottendorf GH (1985) Effect of cephalosporins on organic ion transport in renal membrane vesicles from rat and rabbit kidney cortex. Res Commun Chem Pathol Pharmacol 47: 357–371
Ziegler K, Frimmer M, Fritzsch G, Koepsell H (1990) Cyclosporin binding to a protein component of the renal Na+-d- glucose cotransporter. J Biol Chem 265: 3270–3277
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ullrich, K.J., Rumrich, G., David, C. et al. Bisubstrates: substances that interact with renal contraluminal organic anion and organic cation transport systems. Pflügers Arch 425, 280–299 (1993). https://doi.org/10.1007/BF00374179
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00374179